Kinetic Analysis of the Color of Larch Sapwood and Heartwood during Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Preparation
2.2. Heat Treatment
2.3. Color Measurement
2.4. Kinetics Analysis
2.4.1. Arrhenius Equation and the Time-Temperature Superposition Principle
2.4.2. Zero- and First-Order Reaction
2.5. Data Processing and Analysis
3. Results and Discussion
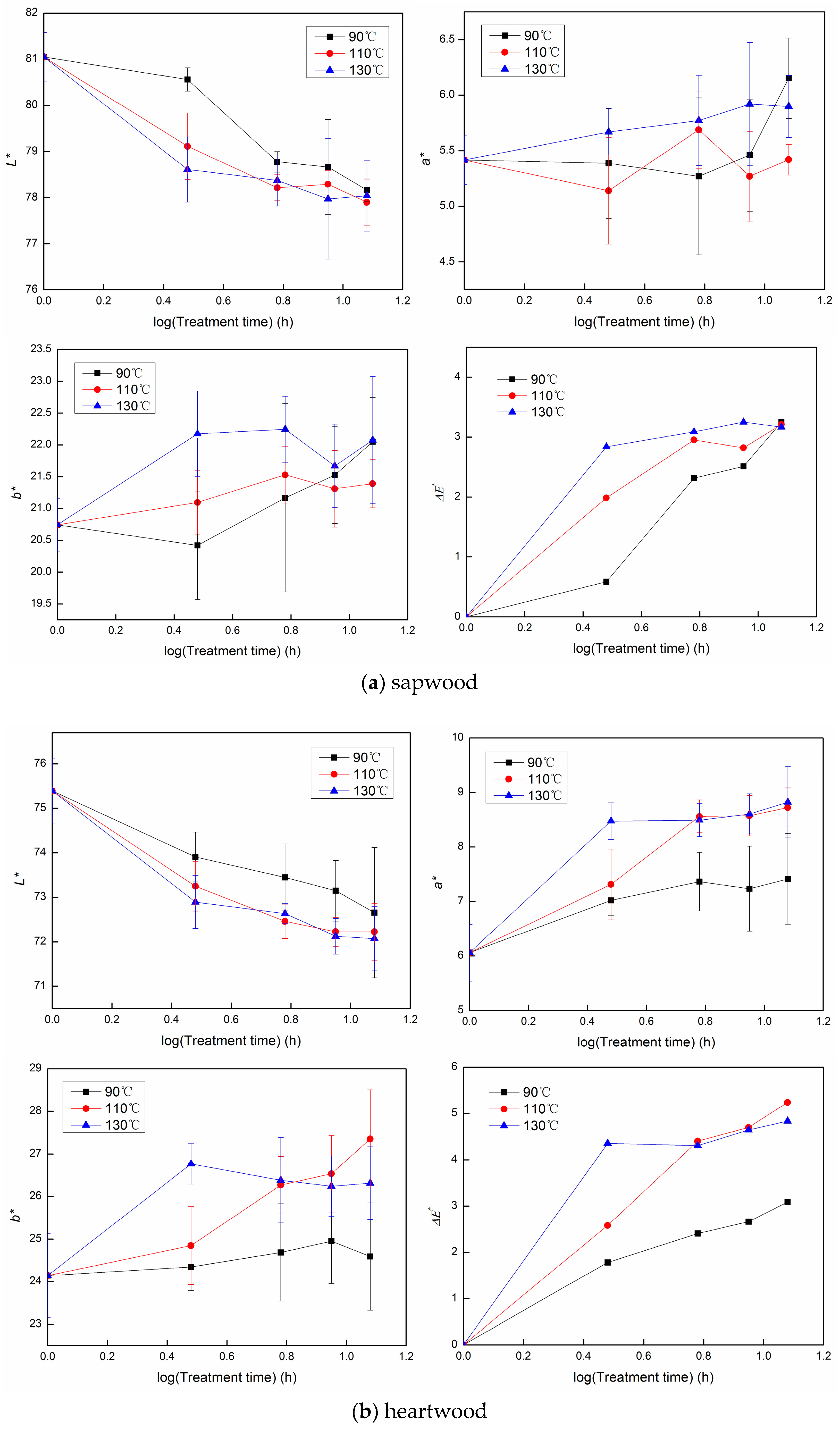
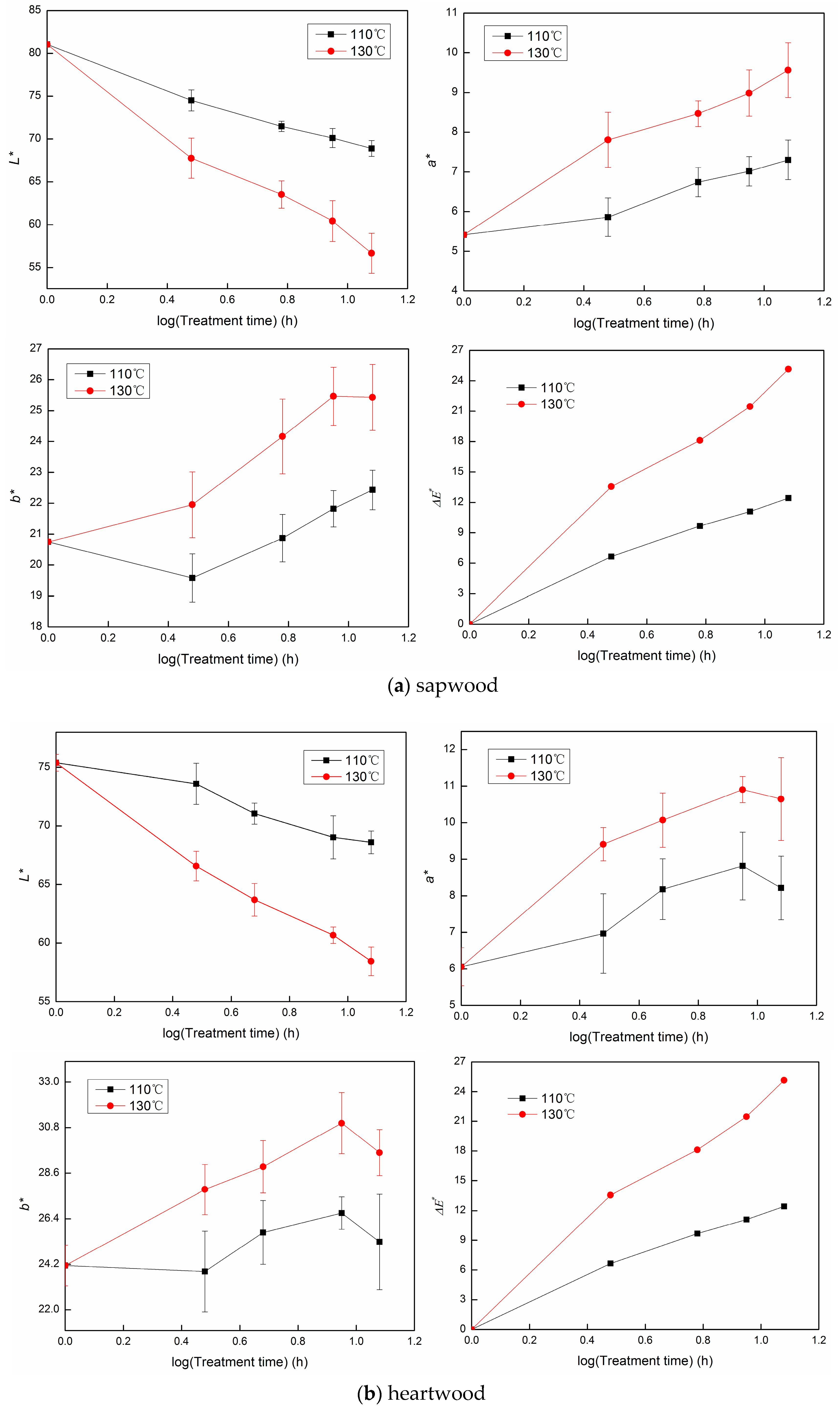
3.1. The Color Change during Heat Treatment
3.2. Kinetics Analysis
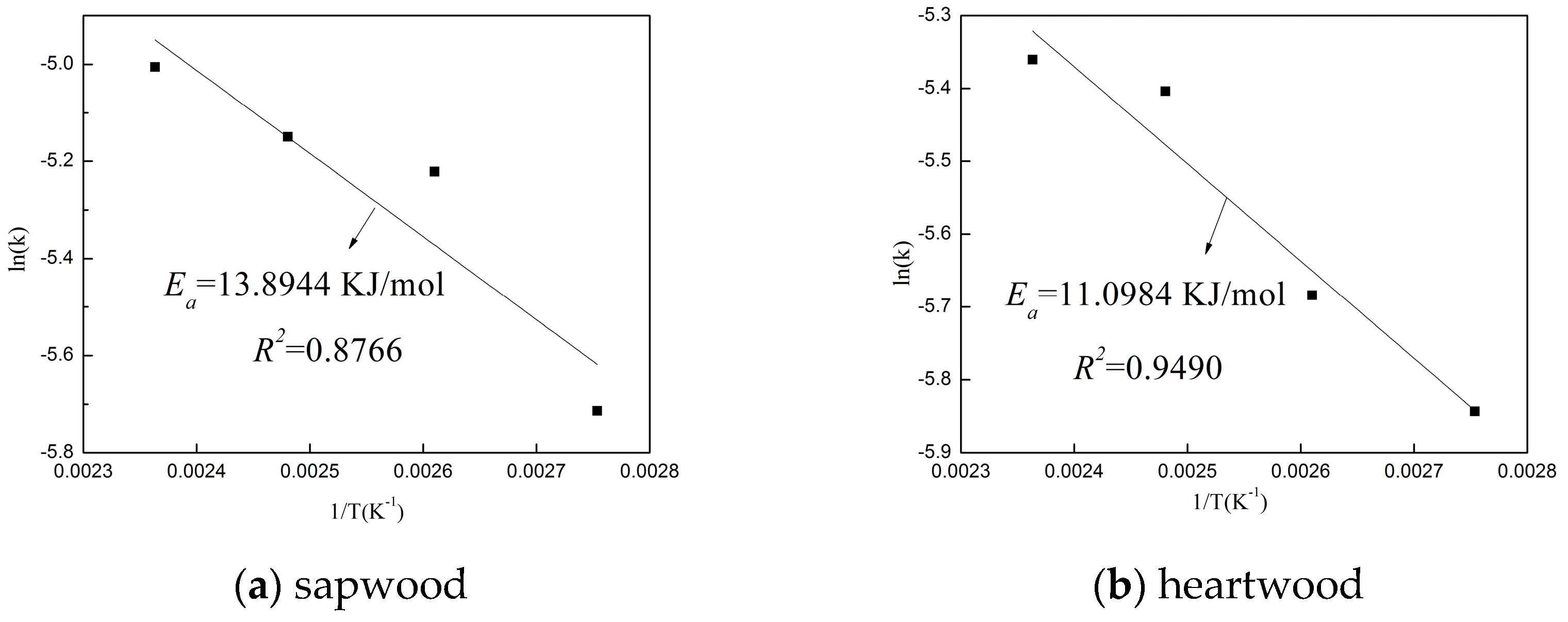
3.2.1. The Time-Temperature Superposition Principle (TTSP)
3.2.2. Zero- and First-Order Reaction
3.2.3. The comparison of the Three Fitting Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pâques, L.E.; García-Casas, M.D.C.; Charpentier, J.P. Distribution of heartwood extractives in hybrid larches and in their related European and Japanese larch parents: Relationship with wood colour parameters. Eur. J. For. Res. 2013, 132, 61–69. [Google Scholar] [CrossRef]
- Montes, C.S.; Hernández, R.E.; Beaulieu, J.; Weber, J.C. Genetic variation in wood color and its correlations with tree growth and wood density of Calycophyllum spruceanum at an early age in the Peruvian Amazon. New For. 2008, 35, 57–73. [Google Scholar] [CrossRef]
- Moya, R.; Berrocal, A. Wood colour variation in sapwood and heartwood of young trees of Tectona grandis and its relationship with plantation characteristics, site, and decay resistance. Ann. For. Sci. 2010, 67, 109. [Google Scholar] [CrossRef]
- Aguilartovar, D.; Moya, R.; Tenorio, C. Wood color variation in undried and kiln-dried plantation-grown lumber of Vochysia guatemalensis. Maderas Ciencia Y Tecnología 2009, 11, 207–216. [Google Scholar]
- Johansson, D.; Morén, T. The potential of colour measurement for strength prediction of thermally treated wood. Holz als Roh-und Werkstoff 2006, 64, 104–110. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačík, F. Colour and chemical changes of the lime wood surface due to CO2 laser thermal modification. Appl. Surf. Sci. 2014, 321, 261–267. [Google Scholar] [CrossRef]
- Brischke, C.; Welzbacher, C.R.; Brandt, K.; Rapp, A.O. Quality control of thermally modified timber: Interrelationship between heat treatment intensities and CIE l*a*b* color data on homogenized wood samples. Holzforschung 2007, 48, 17–22. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Gao, J.; Stark, N.M. The effect of heat treatment on the chemical and color change of black locust (Robinia pseudoacacia) wood flour. Bioresources 2012, 7, 1157–1170. [Google Scholar] [CrossRef]
- Zanuncio, A.J.V.; Carvalho, A.G.; Souza, M.T.D.; Jardim, C.M.; Carneiro, A.D.C.O.; Colodette, J.L. Effect of extractives on wood color of heat treated Pinus radiata and Eucalyptus pellita. Maderas Ciencia Y Tecnologia 2015, 17, 857–864. [Google Scholar] [CrossRef]
- Tuong, V.M.; Li, J. Effect of heat treatment on the change in color. BioResources 2010, 5, 1257–1267. [Google Scholar]
- Sundqvist, B.; Morén, T. The influence of wood polymers and extractives on wood colour induced by hydrothermal treatment. Holz als Roh-und Werkstoff 2002, 60, 375–376. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Zhang, P.; Chen, Y.; Gao, J.; Fan, Y. The role of phenolic extractives in color changes of locust wood (Robinia pseudoacacia) during heat treatment. BioResources 2017, 12, 7041–7055. [Google Scholar]
- Tjeerdsma, B.F.; Militz, H. Chemical changes in hydrothermal treated wood: Ftir analysis of combined hydrothermal and dry heat-treated wood. Eur. J. Wood Wood Prod. 2005, 63, 102–111. [Google Scholar] [CrossRef]
- Brosse, N.; Hage, R.E.; Chaouch, M.; Pétrissans, M.; Dumarçay, S.; Gérardin, P. Investigation of the chemical modifications of beech wood lignin during heat treatment. Polym. Degrad. Stab. 2010, 95, 1721–1726. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Fan, Y.; Tshabalala, M.A.; Stark, N.M. Heat-induced chemical and color changes of extractive-free black locust (Robinia pseudoacacia) wood. Bioresources 2012, 7, 2236–2248. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Gao, J.; Tshabalala, M.A.; Stark, N.M. Spectroscopic analysis of the role of extractives on heat-induced discoloration of black locust (Robinia pseudoacacia). Wood Mater. Sci. Eng. 2012, 7, 209–216. [Google Scholar] [CrossRef]
- Srinivas, K.; Pandey, K.K. Effect of heat treatment on color changes, dimensional stability, and mechanical properties of wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Windeisen, E.; Strobel, C.; Wegener, G. Chemical changes during the production of thermo-treated beech wood. Wood Sci. Technol. 2007, 41, 523–536. [Google Scholar] [CrossRef]
- Kačíková, D.; Kačík, F.; Cabalová, I.; Durkovič, J. Effects of thermal treatment on chemical, mechanical and colour traits in Norway spruce wood. Bioresour. Technol. 2013, 144, 669–674. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.M.; Curling, S.F.; Hale, M.D. On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym. Degrad. Stab. 2009, 94, 2184–2193. [Google Scholar] [CrossRef]
- Lixia, H.; Bo, L.; Shaojin, W. Kinetics of color degradation of chestnut kernel during thermal treatment and storage. Int. J. Agric. Biol. Eng. 2015, 8, 106–115. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, R.Y.; Zhang, Z.T.; Yang, L.W.; Xue, C.H.; Wei, J.; Yang, R.Y. Study on kinetics of color changes in thompson seedless grapes during drying process. Adv. Mater. Res. 2013, 726, 456–462. [Google Scholar] [CrossRef]
- Devi, M.K.; Das, S.K. Kinetics of color changes of popped rice during microwave popping: Effect of salt and moisture content. J. Food Process Eng. 2017, 40, e12560. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Gross, B. Time-temperature superposition principle in relaxation theory. J. Appl. Phys. 1969, 40, 3397. [Google Scholar] [CrossRef]
- Nakano, T. Applicability condition of time-temperature superposition principle (TTSP) to a multi-phase system. Mech. Time-Depend. Mater. 2013, 17, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Jiang, J.; Lu, J.; Cao, J. Application of time-temperature superposition principle to chinese fir orthotropic creep. J. Wood Sci. 2017, 63, 455–463. [Google Scholar] [CrossRef]
- Wang, F.; Huang, T.; Shao, Z. Application of ttsp to wood-development of a vertical shift factor. Holzforschung 2017, 71, 51–55. [Google Scholar] [CrossRef]
- Jabbar, A.; Militký, J.; Kale, B.M.; Rwawiire, S.; Nawab, Y.; Baheti, V. Modeling and analysis of the creep behavior of jute/green epoxy composites incorporated with chemically treated pulverized nano/micro jute fibers. Ind. Crops Prod. 2016, 84, 230–240. [Google Scholar] [CrossRef]
- Matsuo, M.; Umemura, K.; Kawai, S. Kinetic analysis of color changes in cellulose during heat treatment. J. Wood Sci. 2012, 58, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Xing, C.; Pan, H.; Kamdem, P.D.; Matuana, L.M.; Jian, W.; Wang, G. Time-temperature superposition principle application to the hygrothermal discoloration of colored high-density polypropylene/wood composites. Polym. Compos. 2016, 37, 1016–1020. [Google Scholar] [CrossRef]
- Matsuo, M.; Yokoyama, M.; Umemura, K.; Gril, J.; Yano, K.I.; Kawai, S. Color changes in wood during heating: Kinetic analysis by applying a time-temperature superposition method. Appl. Phys. A 2010, 99, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M.; Umemura, K.; Kawai, S. Kinetic analysis of color changes in keyaki (zelkova serrata) and sugi (Cryptomeria japonica) wood during heat treatment. J. Wood Sci. 2014, 60, 12–20. [Google Scholar] [CrossRef]
- Inagaki, T.; Matsuo, M.; Tsuchikawa, S. Nir spectral–kinetic analysis for thermally degraded sugi (Cryptomeria japonica) wood. Appl. Phys. A 2016, 122, 208. [Google Scholar] [CrossRef]
- Matsuo, M.U.; Mitsui, K.; Kobayashi, I.; Kohara, M.; Yoshida, M.; Yamamoto, H. Effect of hygrothermal treatment on wood properties: Color changes and kinetic analysis using four softwood and seven hardwood species. Wood Sci. Technol. 2016, 50, 1145–1160. [Google Scholar] [CrossRef]
- Hu, T.; Sun, L.; Hu, H.; Weise, D.R.; Guo, F. Soil respiration of the dahurian larch (Larix gmelinii) forest and the response to fire disturbance in Da Xing’an Mountains, China. Sci. Rep. 2017, 7, 2967. [Google Scholar] [CrossRef] [PubMed]
- Robert Welzbacher, C.; Brischke, C.; Otto Rapp, A. Influence of treatment temperature and duration on selected biological, mechanical, physical and optical properties of thermally modified timber. Wood Mater. Sci. Eng. 2007, 2, 66–76. [Google Scholar] [CrossRef]
- Santos, D.V.B.D.; Moura, L.F.D.; Brito, J.O. Effect of heat treatment on color, weight loss, specific gravity and equilibrium moisture content of two low market valued tropical woods. Wood Res. 2014, 59, 253–264. [Google Scholar]
- Ma, W.; Qiang, T.; Guo, M. Effect of high temperature heat treatment on color change of imitate precious wood of larch. J. Northeast For. Univ. 2016, 44, 37–41. [Google Scholar]
- Oliveira, R.M.D.; Brisolari, A.; Sales, A.; Gonçalves, D. Wettability, shrinkage and color changes of Araucaria angustifolia after heating treatment. Mater. Res. 2010, 13, 351–354. [Google Scholar] [CrossRef]
- Matsuo, M.; Yokoyama, M.; Umemura, K.; Sugiyama, J.; Kawai, S.; Gril, J.; Kubodera, S.; Mitsutani, T.; Ozaki, H.; Sakamoto, M. Aging of wood: Analysis of color changes during natural aging and heat treatment. Holzforschung 2011, 65, 361–368. [Google Scholar] [CrossRef] [Green Version]







| Oven | Vacuum | Saturated Steam | |||||
|---|---|---|---|---|---|---|---|
| Sapwood | Heartwood | Sapwood | Heartwood | Sapwood | Heartwood | ||
| L* | R2 | 0.9987 | 0.9796 | 0.8180 | 0.9528 | 0.9972 | 0.9754 |
| RMSE | 0.2769 | 0.2005 | 0.2989 | 0.0911 | 0.2735 | 0.8052 | |
| ΔE* | R2 | 0.9641 | 0.9717 | 0.8352 | 0.9108 | 0.9974 | 0.9575 |
| RMSE | 0.2896 | 0.1884 | 0.3168 | 0.2351 | 0.2846 | 1.4888 | |
| Oven | Vacuum | Saturated Steam | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90 °C | 110 °C | 130 °C | 150 °C | 90 °C | 110 °C | 130 °C | 110 °C | 130 °C | ||
| S 1 | k | 0.2638 | 0.4302 | 0.4595 | 0.5270 | 0.2635 | 0.3163 | 0.3226 | 1.1910 | 2.3079 |
| R2 | 0.8753 | 0.9317 | 0.9453 | 0.8892 | 0.9239 | 0.7514 | 0.6896 | 0.8304 | 0.8437 | |
| RMSE | 0.3781 | 0.4589 | 0.4450 | 0.7001 | 0.3605 | 0.6754 | 0.8533 | 1.9636 | 3.6164 | |
| H 2 | k | 0.2165 | 0.2513 | 0.3309 | 0.3473 | 0.2562 | 0.3354 | 0.3457 | 0.6308 | 1.6016 |
| R2 | 0.9458 | 0.9089 | 0.9384 | 0.9123 | 0.8454 | 0.7309 | 0.7127 | 0.9618 | 0.8724 | |
| RMSE | 0.2076 | 0.3040 | 0.3370 | 0.4133 | 0.3989 | 0.7600 | 0.8137 | 0.5262 | 2.2684 | |
| Oven | Vacuum | Saturated Steam | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90 °C | 110 °C | 130 °C | 150 °C | 90 °C | 110 °C | 130 °C | 110 °C | 130 °C | ||
| S 1 | k | −0.1538 | −0.3044 | −0.3224 | −0.3152 | −0.2730 | −0.1182 | −0.0385 | −0.6252 | −1.2699 |
| R2 | 0.9298 | 0.9634 | 0.9794 | 0.9874 | 0.9218 | 0.8924 | 0.8894 | 0.9623 | 0.9958 | |
| RMSE | 0.1739 | 0.2172 | 0.1641 | 0.1228 | 0.3403 | 0.2318 | 0.1023 | 0.4538 | 0.2780 | |
| H 2 | k | −0.1624 | −0.1716 | −0.2259 | −0.2808 | −0.1391 | −0.2759 | −0.0594 | −0.5953 | −0.9490 |
| R2 | 0.9942 | 0.9996 | 0.9998 | 0.9808 | 0.9754 | 0.9146 | 0.9113 | 0.9132 | 0.9757 | |
| RMSE | 0.0420 | 0.0106 | 0.0103 | 0.1372 | 0.0783 | 0.3763 | 0.0846 | 0.8263 | 0.5305 | |
| Oven | Vacuum | Saturated Steam | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90 °C | 110 °C | 130 °C | 150 °C | 90 °C | 110 °C | 130 °C | 110 °C | 130 °C | ||
| S 1 | k | 0.0033 | 0.0054 | 0.0058 | 0.0067 | 0.0033 | 0.0040 | 0.0067 | 0.0158 | 0.0331 |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | |
| RMSE | 0.0047 | 0.0056 | 0.0054 | 0.0086 | 0.0045 | 0.0084 | 0.0107 | 0.0247 | 0.0448 | |
| H 2 | k | 0.0029 | 0.0034 | 0.0045 | 0.0047 | 0.0035 | 0.0045 | 0.0047 | 0.0087 | 0.0236 |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | |
| RMSE | 0.0027 | 0.0040 | 0.0044 | 0.0054 | 0.0053 | 0.0102 | 0.0109 | 0.0071 | 0.0298 | |
| Oven | Vacuum | Saturated Steam | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90 °C | 110 °C | 130 °C | 150 °C | 90 °C | 110 °C | 130 °C | 110 °C | 130 °C | ||
| S 1 | k | −0.0771 | −0.0881 | −0.0873 | −0.0775 | −0.1742 | −0.0466 | −0.0127 | −0.0671 | −0.0674 |
| R2 | 0.9762 | 0.9994 | 0.9988 | 0.9988 | 0.9269 | 0.9739 | 0.9814 | 0.9990 | 0.9999 | |
| RMSE | 0.0941 | 0.0272 | 0.0384 | 0.0525 | 0.3246 | 0.0961 | 0.0337 | 0.0693 | 0.0349 | |
| H 2 | k | −0.9844 | −0.0818 | −0.0744 | −0.0810 | −0.0584 | −0.0730 | −0.0129 | −0.1391 | −0.0679 |
| R2 | 0.9996 | 0.9979 | 0.9988 | 0.9967 | 0.9907 | 0.9886 | 0.9941 | 0.9906 | 0.9998 | |
| RMSE | 0.0110 | 0.0264 | 0.0262 | 0.0556 | 00471 | 0.1207 | 0.0188 | 0.2352 | 0.0497 | |
| TTSP | Zero-Order Reaction | First-Order Reaction | |
|---|---|---|---|
| Sapwood | 0.8180 < R2 < 0.9987 | 0.6896 < R2 < 0.9453 | 0.9999 |
| Heartwood | 0.9528 < R2 < 0.9796 | 0.7127 < R2 < 0.9618 | 0.9999 |
| TTSP | Zero-Order Reaction | First-Order Reaction | |
|---|---|---|---|
| Sapwood | 0.8352 < R2 < 0.9974 | 0.8724 < R2 < 0.9958 | 0.9269 < R2 < 0.9999 |
| Heartwood | 0.9108 < R2 < 0.9717 | 0.9113 < R2 < 0.9998 | 0.9886 < R2 < 0.9998 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhang, P.; Liu, Y.; Chen, Y.; Gao, J.; Fan, Y. Kinetic Analysis of the Color of Larch Sapwood and Heartwood during Heat Treatment. Forests 2018, 9, 289. https://doi.org/10.3390/f9060289
Wei Y, Zhang P, Liu Y, Chen Y, Gao J, Fan Y. Kinetic Analysis of the Color of Larch Sapwood and Heartwood during Heat Treatment. Forests. 2018; 9(6):289. https://doi.org/10.3390/f9060289
Chicago/Turabian StyleWei, Yanxia, Peng Zhang, Yang Liu, Yao Chen, Jianmin Gao, and Yongming Fan. 2018. "Kinetic Analysis of the Color of Larch Sapwood and Heartwood during Heat Treatment" Forests 9, no. 6: 289. https://doi.org/10.3390/f9060289




