1. Introduction
Anthropogenic emission of greenhouse gasses and concomitant global climate changes have fostered an impetus for the modeling and quantifying of global carbon stocks and balances within terrestrial ecosystems. The overall carbon balance of an ecosystem is dependent upon carbon assimilation (
i.e., photosynthesis) being larger in magnitude than the carbon lost from ecosystem respiration, oxidation from fire, and carbon removal (e.g., biomass harvesting, herbivory, run-off, and leaching) [
1]. Soil heterotrophic respiration (R
h) is the largest heterotrophic component of ecosystem respiration and is often compared with net primary productivity to estimate whether forests are carbon sinks or sources [
1,
2]. Carbon sources for R
h from free-living soil microbes include soil organic matter, dead plant matter, and exudates from roots and R
h thus occurs in both root-free bulk soil and the root-affected rhizosphere [
3]. In some woody species, including pines, CO
2 is also released by the metabolism of root exudates by ectomycorrhizal symbionts [
4,
5]. Mycorrhizally sourced CO
2 is also a component of R
h, however because of the interaction between roots and microbes in the rhizosphere, partitioning total R
s into its autotrophic (
i.e., root-derived) and heterotrophic components is challenging.
Methods that have been developed to partition R
s each have inherent strengths and weaknesses [
3,
6]. One common technique uses trenching to sever existing roots in order to cut off the photosynthetic carbon allocation pathway, which then theoretically leaves residual heterotrophic activity in the soil and allows for direct measurement of R
h [
7]. With this technique, plots that are trenched are often fairly large and in place for a year or more before R
h is measured [
8]. The downside of using root exclusion techniques such as trenching includes: (1) modification to the soil environment due to changes in evapotranspirational demand from root-severed soil and run-off and leaching patterns; (2) an increase in R
h due to the newly deceased root tissues in the root-severed soil; (3) residual autotrophic respiration (R
a) may continue in severed roots with large carbohydrate reserves; and (4) eventual changes in the microbial community composition as labile substrates diminish and microbes that decompose recalcitrant organic material become more dominant [
3,
6,
7,
8,
9]. In order to reduce some of these artifacts, a technique to measure R
h from root-excluded soils utilizing small-diameter root exclusion tubes has been developed in which root exclusion tubes are pushed through otherwise undisturbed soil, severing the existing roots and excluding root ingrowth [
8]. Although some of the methodological artifacts of trenching remain a limitation with root exclusion tubes, such as potential changes to the soil hydrology, small root exclusion tubes are easier to install than the effort and expense necessary for trenching and they can be installed with higher replication and staggered over time to determine how the profile of R
h changes seasonally or in response to disturbances. In addition, the smaller volume of root-excluded soil as compared to trenching should reduce the total available carbohydrate reserves and allow R
h to be more readily estimated after installation, thus reducing the potential for changes in soil environment and microbial community composition [
8,
10].
The objective of this study was to estimate the proportion of R
h to R
s in three longleaf pine (
Pinus palustris Mill. (
P. palustris)) plantations with small-diameter root exclusion tubes. Net primary productivity and related standing carbon stocks of longleaf pine forests have been previously examined e.g., [
11,
12,
13]; however, relatively less is known about the magnitude of R
h in longleaf pine forests, thus hindering accurate estimates of the carbon balance of longleaf pine forests. Specifically, the proportion of R
h to total R
s has been estimated in only one known longleaf pine study (85% to 96%) [
14]. We hypothesized, based on the previous longleaf pine study [
14] and research in another southern conifer, loblolly pine (
Pinus taeda L. (
P. taeda)) [
10], that R
h would comprise 70% to 90% of R
s. Root exclusion tubes were installed in May 2013 in three 26-year-old longleaf pine stands in western Georgia and compared with paired control R
s measurements to determine the proportion of R
h to total R
s. A recent methodologically similar study with loblolly pine demonstrated that root carbohydrates are metabolized rapidly after severing, and that R
h estimates from root-excluded soil stabilized after 41 days [
10]. We attempted to reduce the impacts of residual starch reserves in longleaf pine roots by installing the root exclusion treatment in mid-May during a period of starch depletion [
15], and we hypothesized that by late August (~100 days), measurements of R
s from root exclusion tubes would provide a relatively stable estimate of R
h. Because of the excessively drained, sandy soils present in these stands [
16], we expected minimal effect of root exclusion tubes on soil moisture. When trenching or small root exclusion tubes are coupled with root biomass measurements, the amount of root decay that has occurred post-treatment can be quantified and used to recalculate more accurate estimates of R
h [
7,
17]. Thus, we also incorporated an estimation of fine root decay into R
h based upon measurements of root biomass in both the root-excluded and control soil profiles to ameliorate potential impacts of artificially enhanced carbohydrate metabolism after severing.
2. Experimental Section
2.1. Study Site
This study was conducted in three 26-year-old longleaf pine stands located on property in a conservation easement held by The Nature Conservancy within the Chattahoochee Fall Line Wildlife Management area in Talbot County, Georgia, USA (32°34′ N & −84°30–36′ W;
Table 1). These stands were located within the Sand Hills Ecoregion, which is characterized by dry, deep sand and gently rolling hills that are excessively drained and nutrient poor [
18]. All three stands were planted in 1988 at a density of 1793 seedlings ha
−1 and spacing of 1.8 m by 3.0 m, and no known thinning occurred after planting. The 30-year mean annual air temperature for this area is 24.6 °C with mean January and July air temperature of 8.4 °C and 28.1 °C, respectively [
19]. The 30-year mean precipitation is 1180 mm, spread evenly throughout the year. Stands 1 and 2 were located in Lakeland sand soil (2% to 5% slopes, excessively drained) and Stand 3 was located in a Troup loamy sand soil (2% to 5% slopes, somewhat excessively drained) [
16]. Longleaf pine basal area measured in the experimental plots ranged from 17.3 to 21.4 m
2·ha
−1 and density ranged from 978 to 1567 trees·ha
−1 (
Table 1). Mean diameter at breast height (1.37 m, DBH) of longleaf pine trees ranged from 11.5 to 15.4 cm. Basal area, density, and mean DBH of hardwoods in the stands ranged from 0.0 to 0.2 m
2·ha
−1, 0 to 89 trees·ha
−1, and 4.0 to 4.4 cm, respectively. Understory vegetation was sparse and consisted mainly of bunch grasses, saw palmetto (
Serenoa repens (W. Bartram) Small), prickly pear cacti (
Opuntia spp.), and some poison oak (
Toxicodendron pubescens Mill.). Stands were last burned about one year prior to this study and have been maintained on a frequent (1–3 year) burning regime under The Nature Conservancy’s management. No burn records were available previous to the easement held by The Nature Conservancy.
Table 1.
Location and forest structural characteristics of the three 26-year-old longleaf pine (LLP; Pinus palustris Mill.) stands in Talbot County, Georgia, USA. Note: HW, hardwoods; DBH, diameter at breast height (1.37 m).
Table 1.
Location and forest structural characteristics of the three 26-year-old longleaf pine (LLP; Pinus palustris Mill.) stands in Talbot County, Georgia, USA. Note: HW, hardwoods; DBH, diameter at breast height (1.37 m).
| | Stand 1 | Stand 2 | Stand 3 |
|---|
| | Location & Size |
| ° N | 32°34.469′ | 32°34.716′ | 32°34.706′ |
| ° W | −84°36.039′ | −84°31.371′ | −84°30.117′ |
| Size (ha) | 75.3 | 4.0 | 2.4 |
| | Basal Area (m3·ha−1) |
| Total | 18.82 | 21.48 | 17.34 |
| LLP | 18.60 | 21.40 | 17.34 |
| HW | 0.22 | 0.08 | 0.00 |
| | Tree Density (trees·ha−1) |
| Total | 1222 | 1533 | 1922 |
| LLP | 978 | 1089 | 1567 |
| HW | 89 | 33 | 0 |
| Dead | 156 | 411 | 356 |
| | Mean DBH ± SE (cm) |
| LLP | 14.89 ± 0.48 | 15.44 ± 0.35 | 11.50 ± 0.25 |
| HW | 4.39 ± 1.36 | 4.00 ± 2.70 | na |
2.2. Sampling Design
In 2013, one 30 m by 30 m experimental plot was established in each of the three stands, and 10 trees were randomly selected from within each stand from a gridded identification system based on row and tree number. At each tree, a temporary R
s collar (PVC, 10 cm diameter, 4.5 cm height) was placed at 1.0 m from that tree at a randomly chosen 45° angle, and a paired temporary R
s collar was also placed 1.0 m from an adjacent tree at an angle parallel to the first (
Figure 1a). Litter was cleared away from the location of collar placement and the collars were gently pounded into the soil to a depth of 2.0 cm below mineral soil. Temporary R
s collar pairs were installed on 10 May and 15 May 2013 and used for initial, pretreatment R
s measurements 24 h after installation.
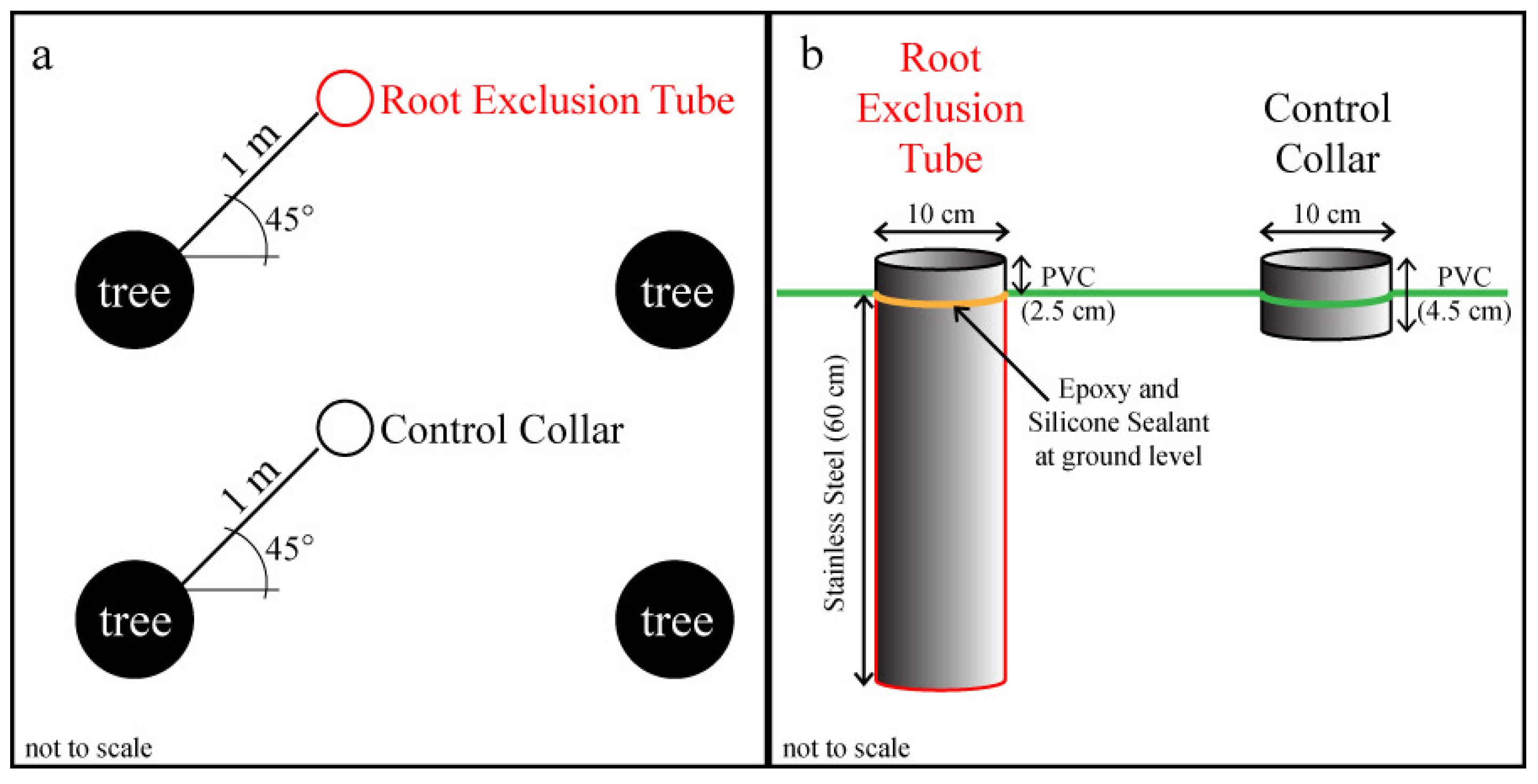
Figure 1.
Experimental design schematics showing: (a) the random placement of root exclusion tubes and control collars 1 m from trees at a 45° angle from the tree rows; and (b) the size and composition of the root exclusion tubes and control collars. This study was conducted in three longleaf pine (Pinus palustris Mill.) stands.
Figure 1.
Experimental design schematics showing: (a) the random placement of root exclusion tubes and control collars 1 m from trees at a 45° angle from the tree rows; and (b) the size and composition of the root exclusion tubes and control collars. This study was conducted in three longleaf pine (Pinus palustris Mill.) stands.
After initial R
s measurements were made on the temporary R
s collars, root exclusion tubes were installed at the location of one randomly selected temporary R
s collar from each pair. Root exclusion tubes were constructed from a 10 cm diameter stainless steel tube cut to 60 cm length with a sharp, beveled bottom edge (
Figure 1b). A small pretreatment test of 1 meter-long root exclusion tubes in an adjacent longleaf pine stand illustrated that 60 cm should be deep enough to exclude all herbaceous and lateral longleaf pine roots and that the beveled bottom edge was able to cut through roots up to about 5 cm in diameter. During installation, tubes were pounded into the ground with a rubber mallet and a board until the tube edge was approximately 2 cm above the soil surface. A 2.5 cm long, 10 cm diameter PVC collar was glued to the exposed top of each root exclusion tube with instant epoxy, and waterproof silicone sealant was applied to create an airtight seal (
Figure 1b). After the epoxy and silicone had set, the root exclusion tubes were gently pushed down into the soil until the metal tube edge was flush with the adjacent undisturbed soil, leaving 2.5 cm of PVC collar aboveground. The remaining ten temporary collars per plot became permanent control treatment collars (
i.e., no root exclusion). All root exclusion tubes were installed on 14 or 16 May 2013. Litter was removed and live vegetation was clipped from within all collars as needed to maintain bare soil.
2.3. Soil Respiration Measurements
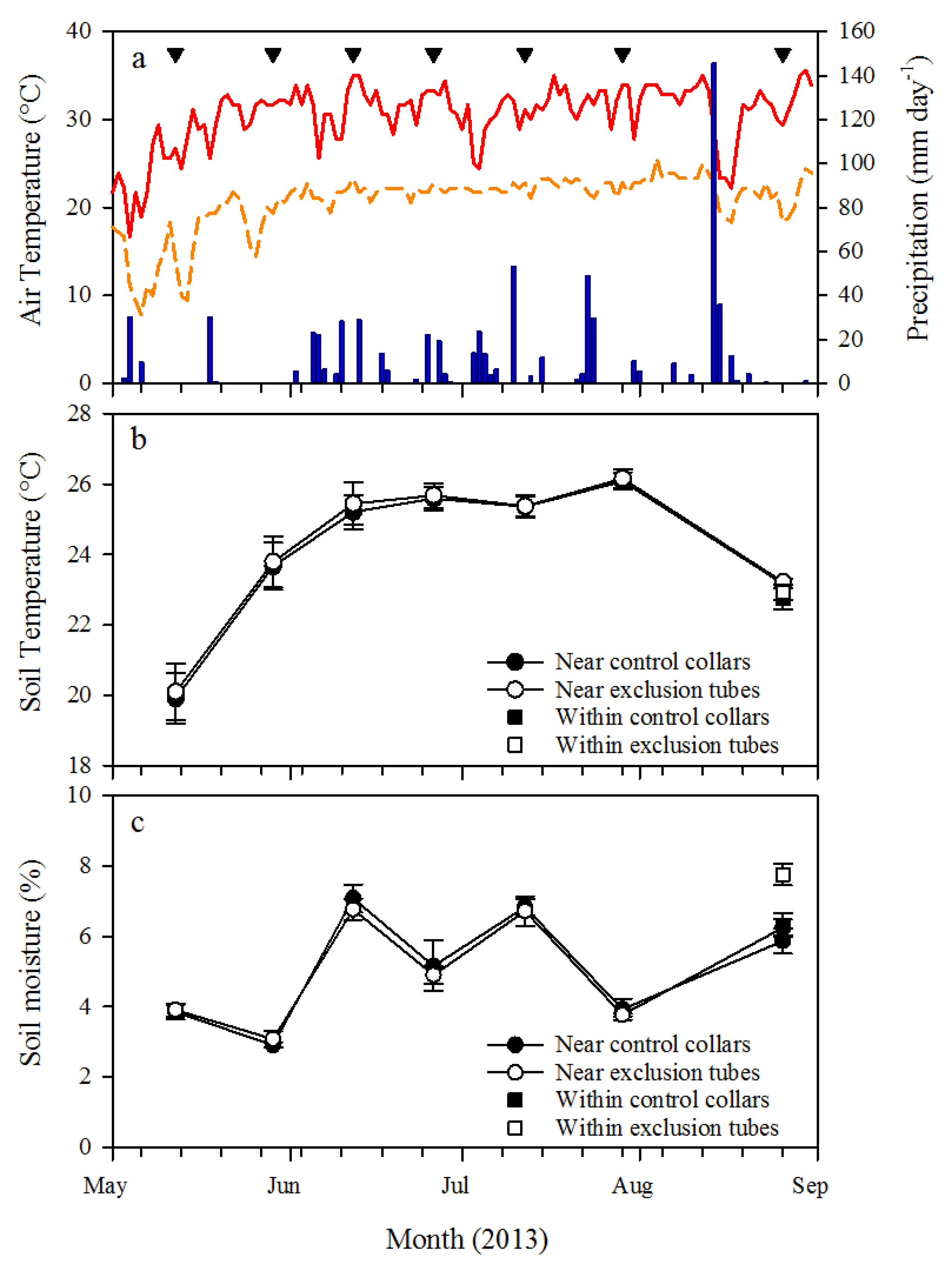
Soil respiration was measured on both root exclusion collars and control collars by placing a LI-COR 6400-09 soil respiration chamber connected to a LI-COR 6400 infrared gas analyzer (LI-COR Biosciences, Lincoln, NE, USA) on the portion of PVC located above the ground. A foam gasket was used on the soil respiration chamber to create an airtight seal with the collars and depth to soil surface within each collar was used to calculate chamber volume for Rs measurements. Ambient CO2 concentration (CO2amb) was measured at the first block within each plot. Carbon dioxide was scrubbed from the chamber air by passing it through soda lime, and then CO2amb was logged with the LI-COR 6400 console over at least 30 intervals from 5 ppm below ambient CO2amb to 5 ppm above ambient CO2amb. Soil respiration was then derived from linear regression of the logged intervals. Soil respiration was measured at approximately 2-week intervals from 10 May 2013 (first pretreatment measurement) through 26 August 2013; with the exception of mid-August because of heavy rains. All Rs measurements were made from 8:30 a.m. to 1:00 p.m., and the measurement sequence of the plots and blocks were randomly determined each day. Soil temperature and volumetric soil moisture were measured within 10 cm of the collars at the time of Rs measurements with a 15 cm depth soil temperature probe connected to the LI-COR 6400 and a 20 cm depth time domain reflectometry soil moisture probe (Hydrosense II, Campbell Scientific, Inc., Logan, UT, USA), respectively. After the final Rs measurement was made at each collar on 26 August 2013, soil moisture and soil temperature were measured within each collar to test for treatment effects on the soil environment.
2.4. Soil Sampling
Following the final Rs measurements, soil samples were removed from directly underneath the control collars with a 10 cm diameter soil auger at depths of 0–15 cm, 15–30 cm, 30–45 cm, and 45–60 cm and placed in plastic bags for transport. Root exclusion collars and tubes were pulled out of the ground, sealed with plastic, and transported to the lab for processing. In the laboratory, soil was removed from root exclusion tubes in sections of 15 cm lengths to correspond with soil depths of 0–15 cm, 15–30 cm, 30–45 cm, and 45–60 cm. All soil was air dried and sifted through a 2 mm sieve. Roots were removed, washed, and sorted into fine and coarse size classes (diameter ≤ 2 mm or diameter > 2 mm, respectively) and live versus dead based on texture, color and resiliency. Roots were oven dried at 70 °C and weighed.
2.5. Data Analysis
The experimental design was a randomized complete block design with collar pairs considered as blocks (n = 30) and individual collars considered the experimental unit. To account for random variation between stands and between blocks, treatment effects (i.e., root exclusion tubes versus control Rs collar only) were analyzed with a mixed-model approach. Data were analyzed across all measurement replications with date as a random factor to measure the overall treatment effect and at each measurement date to determine the timing at which the root exclusion tubes were significantly different than the control collars. The initial, pretreatment Rs measurements were pooled for analysis. All statistical analysis was done with SAS (version 9.3, SAS Institute Inc., Cary, NC, USA) and with a significance level of α = 0.05.
Soil respiration measurements from the control collars were used to estimate total Rs, whereas soil respiration measurements from the root exclusion tubes were used to estimate Rh. In addition to direct measurements of root exclusion tube Rs to estimate Rh, Rs from root exclusion tubes was also corrected for pretreatment Rs variation between collar pairs within blocks and for increased soil moisture and root decay caused by the presence of root exclusion tubes. Root exclusion tube Rh was corrected for pretreatment Rs variation by using the initial within-block difference in Rs as an offset.
The respiration measured from exclusion tubes was corrected for any soil moisture differences between treatments as described by Prolingheuer
et al. [
20]. The relationship between R
s from root exclusion tubes and soil moisture on the final day was used to correct R
h based on the volumetric soil moisture measurements made near the root exclusion tubes. Specifically, the difference between soil moisture measured within and adjacent to root exclusion tubes was used to calculate the expected change in R
s due to root exclusion using the fitted relationship between R
s and soil moisture.
Fine root decay was estimated at the block-level using a modified exponential decay function,
M1 =
M0 exp(−
kt), where
M1 is the fine root biomass in the root exclusion tubes,
M0 is the fine root biomass below the control collars,
k is the decay rate, and
t is the incubation time [
7]. The total loss of live fine root biomass was stoichiometrically converted to μmol·CO
2·m
−2·s
−1 assuming fine roots were 50% carbon by mass [
21]. Total live fine root biomass over the entire sampled soil profile (0 to 60 cm depth) was compiled and used for these calculations.
4. Discussion
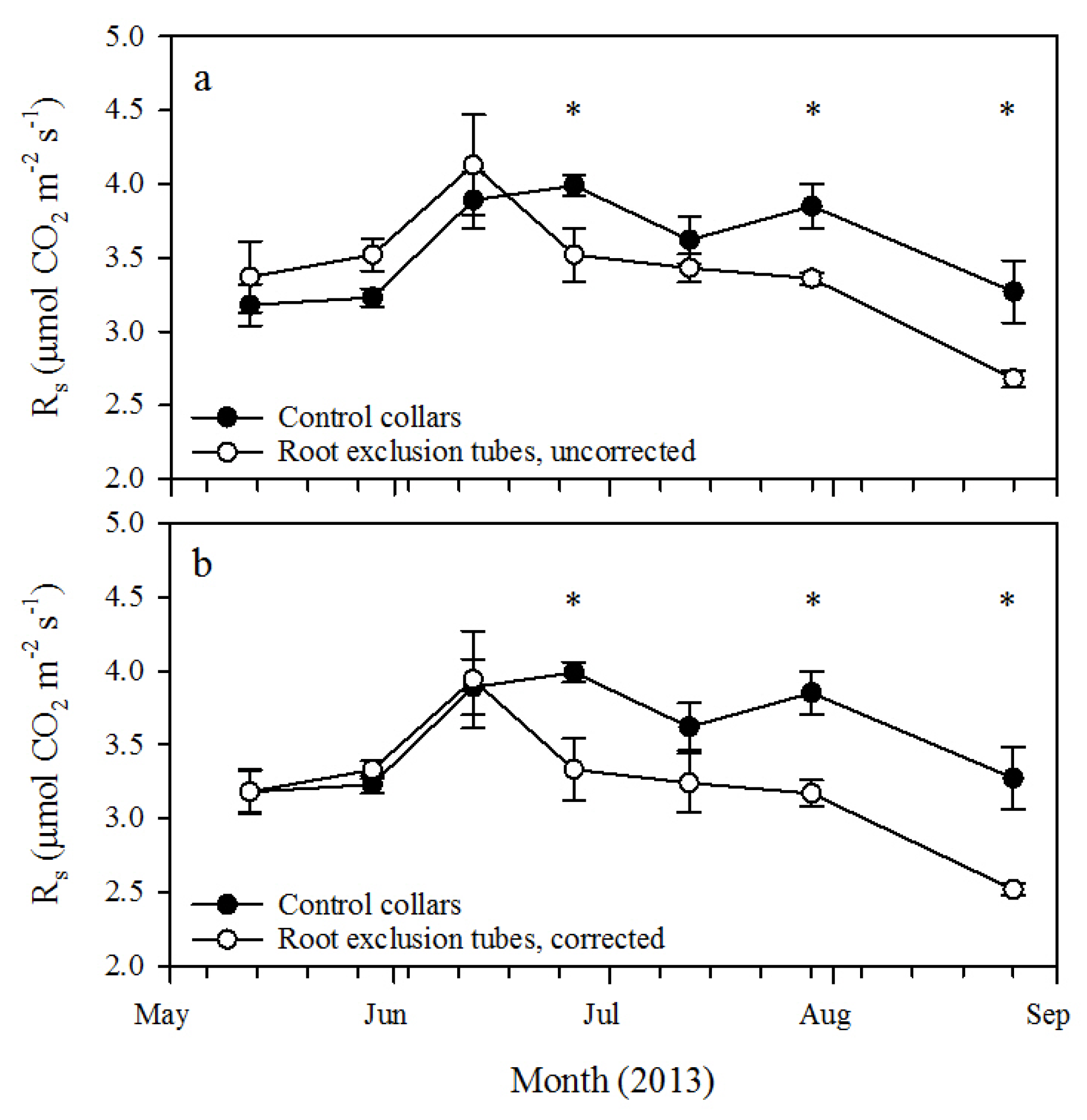
This study utilized small-diameter root exclusion tubes for relatively rapid estimation of R
h in 26-year-old longleaf pine forests in western Georgia. After about 40 days of treatment (
i.e., by 26 June 2013), R
s measured from the root exclusion tubes was significantly lower than R
s from the control collars. At that time, the corresponding ratio of R
h to R
s was 83.5% ± 5.5% if R
h was corrected for pretreatment R
s variation and 88.2% ± 4.8% without correction. This is higher, but not substantially different, than the uncorrected ratio measured at the final measurement date, about 100 days after root exclusion tube installation (
i.e., by 26 August 2013). Heim
et al. [
10] similarly found that after 41 days, root exclusion tubes significantly reduced R
s compared to adjacent, undisturbed soil in a nine-year-old loblolly pine plantation with a corresponding uncorrected ratio of R
h to R
s of 79.0%. The results of these two studies indicate that 40 to 50 days of root exclusion may be an adequate time period to isolate R
h from root exclusion treatment tubes.
Rapid measurements of R
s from root severed or trenched soils are recommended by Sayer and Tanner [
23] to reduce methodological artifacts such as root decomposition or ingrowth, soil moisture alteration, and microbial community composition changes. Likewise, Vogel and Valentine [
8] suggest that measurements of R
s from small root exclusion tubes within three weeks of installation may mitigate effects of root exclusion on soil moisture. However, three weeks of post-treatment incubation was insufficient to detect R
h in longleaf pine, perhaps because of greater carbohydrate stores in longleaf pine roots relative to other species [
24,
25]. We suggest that the longer incubation did not profoundly change soil moisture dynamics or our overall estimate of R
h to R
s, because soils in this study were sandy with excessive drainage and the presence of the root exclusion tubes increased soil moisture on the final measurement date by only about 1%. On the other hand, the impact of root severing on the soil microbial community composition and the rate of R
h from ectomycorrhizae associated with longleaf pine roots was untested in this study, so further research is recommended to determine the incubation timeframe that minimizes treatment effects on the soil microbes, including ectomycorrhizae.
Only two known direct estimates of R
h or R
a in longleaf pine forests have been previously published, one with a novel buried root isolation chamber for
in situ measurement of R
a that cannot be compared to this study as they did not partition R
s into its autotrophic and heterotrophic components [
26]. Rather, Cheng
et al. [
26] directly measured R
a on a per root mass basis. The other study provided estimates of the ratio of R
h to R
s based on comparison between root exclusion tubes and control collars after 45 days post-treatment in longleaf pine forests in Georgia [
14]. Collins [
14] measured R
h with shallow 10 cm deep root exclusion tubes and found that R
h to R
s ranged from 90% to 96% in longleaf pine stands on sandy soils and from 85% to 88% in stands on clayey soils, compared to our 60 cm deep tubes and uncorrected ratio of 82%. In our study, 80% of live roots were in the top 30 cm of the soils and live roots were present down to 60 cm. As such, the relatively shallow root exclusion technique of Collins [
14] may have overestimated R
h compared to our estimates.
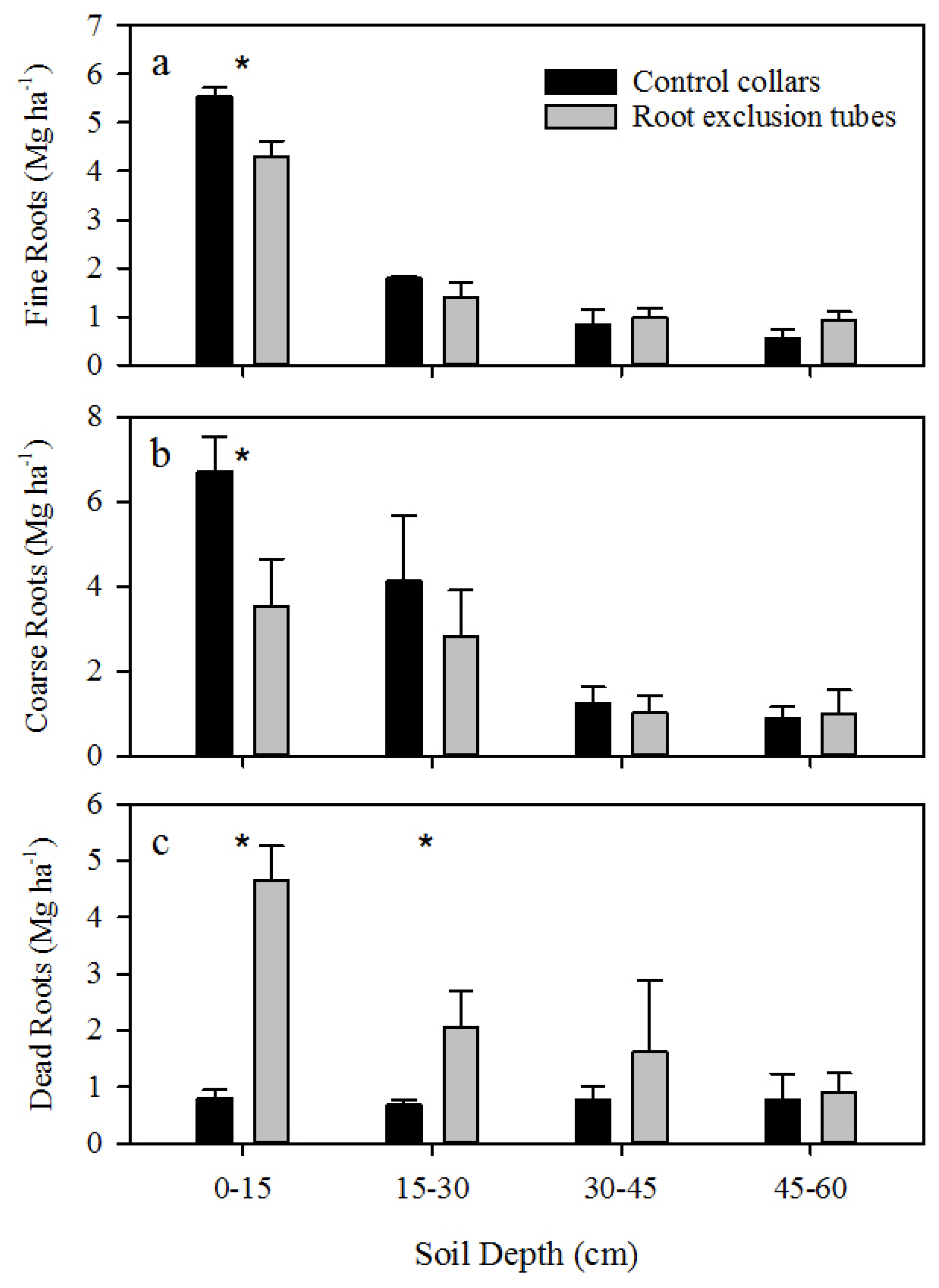
A significant decrease in shallow live roots and increase in shallow dead roots was observed within root exclusion tubes, and not accounting for CO
2 efflux from the death and decay of severed roots may therefore overestimate R
h [
7]. Incorporating an estimate of root decay (
k = 0.52 year
−1) resulted in an approximate 15% decrease in the proportion of R
h to R
s. Accounting for root decay resulted in a 2% to 24% reduction in R
h to R
s ratios in previous studies reviewed by Subke
et al. [
7], and root decay rates cited in Subke
et al. [
7] ranged from
k = 0.14 to 0.96 year
−1 (mean
k = 0.32 year
−1). Studies in deciduous ecosystems generally had higher cited root decay rates and larger decreases in the ratio of R
h to R
s after incorporating root decay than coniferous ecosystem studies [
7]. Root decay rates cited in other R
s partitioning studies not reviewed by Subke
et al. [
7] were
k = 0.5 year
−1 and
k = 0.3 year
−1 for a boreal black spruce (
Picea mariana (Mill.) Britton, Stearns & Poggenb.) forest [
17,
27] and mountain Norway spruce (
Picea abies (L.) Karst.) forest [
9], respectively. Silver
et al. [
28] found that the assumed decomposition rate, which is the percentage of root C that gets oxidized and respired as CO
2, can affect the estimated reduction in R
h to R
s ratio after incorporating root decay. We assumed a decomposition fraction of 1.00, like most studies reviewed by Subke
et al. [
7], but this may overestimate the fraction of root C that is respired. For instance, Heim
et al. [
10] found at most a 60% reduction in soluble loblolly pine root nonstructural carbohydrates 90 days after severing.
Subke
et al. [
7] performed a global meta-analysis of the proportion of R
h to R
s based on ecosystem type, biome, stand age, and R
h partitioning technique (e.g., root exclusion including trenching). Subke
et al. [
7] and others [
29,
30] found that the proportion of R
h to R
s varies with annual R
s levels. To make a comparison to Subke
et al. [
7], we assumed that annual R
s from these longleaf pine stands was similar to those measured in other nearby longleaf pine stands varying in age and structure (12 to 14 Mg·C·ha
−1·year
−1) [
31]. Our range in R
h to R
s corrected for root decay is comparable to the mean ratio of R
h to R
s (60% ± 12%) from Subke
et al. [
7] across similar temperate coniferous ecosystems with annual R
s from 12 to 14 Mg·C·ha
−1·year
−1. On the other hand, our root decay-corrected ratio is high compared against the mean ratio of R
h to R
s from studies that corrected for root decay in coniferous ecosystems (47%) [
7]. However, Subke
et al. [
5] discusses how the majority of root decay-corrected ratios were derived from boreal forests, causing a bias towards lower mean R
h to R
s ratios than would be expected from temperate coniferous ecosystems.
The specific conditions of these longleaf pine ecosystems made this study ideal for a “proof-of-concept” examination of small-diameter root exclusion tubes to partition total belowground R
s into its heterotrophic and autotrophic components. These stands are managed with frequent prescribed burns (every 1–3 years) resulting in negligible litter layers and a reduced understory. Therefore, microbial respiration from the litter layer and herbaceous ingrowth in the root exclusion tubes were minimized, which are both potential sources of error in root exclusion studies [
8,
23]. As previously discussed, soils in these stands are deep sands, which facilitated the installation of the steel tubes to deeper depths than possible in more clayey soils and reduced the treatment effects on soil compaction and soil moisture. Under these conditions, root exclusion tubes decreased R
s by 18% to 34% and provided an estimate of longleaf pine R
h. However, because estimates of R
h are often specific to the ecosystem and ecological context (e.g., season, soil types, humus form, vegetation composition, management regimes,
etc.) [
2,
7], further research across the native range of longleaf pine, which encompasses a range in soil types, understory composition, and climate, is necessary to gain a more complete understanding of the heterotrophic and autotrophic components of R
s in longleaf pine ecosystems.