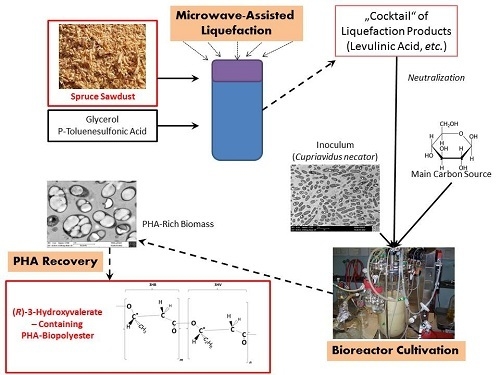
Liquefied Wood as Inexpensive Precursor-Feedstock for Bio-Mediated Incorporation of (R)-3-Hydroxyvalerate into Polyhydroxyalkanoates
Abstract
:1. Introduction
2. Results
2.1. Shake Flask Experiment
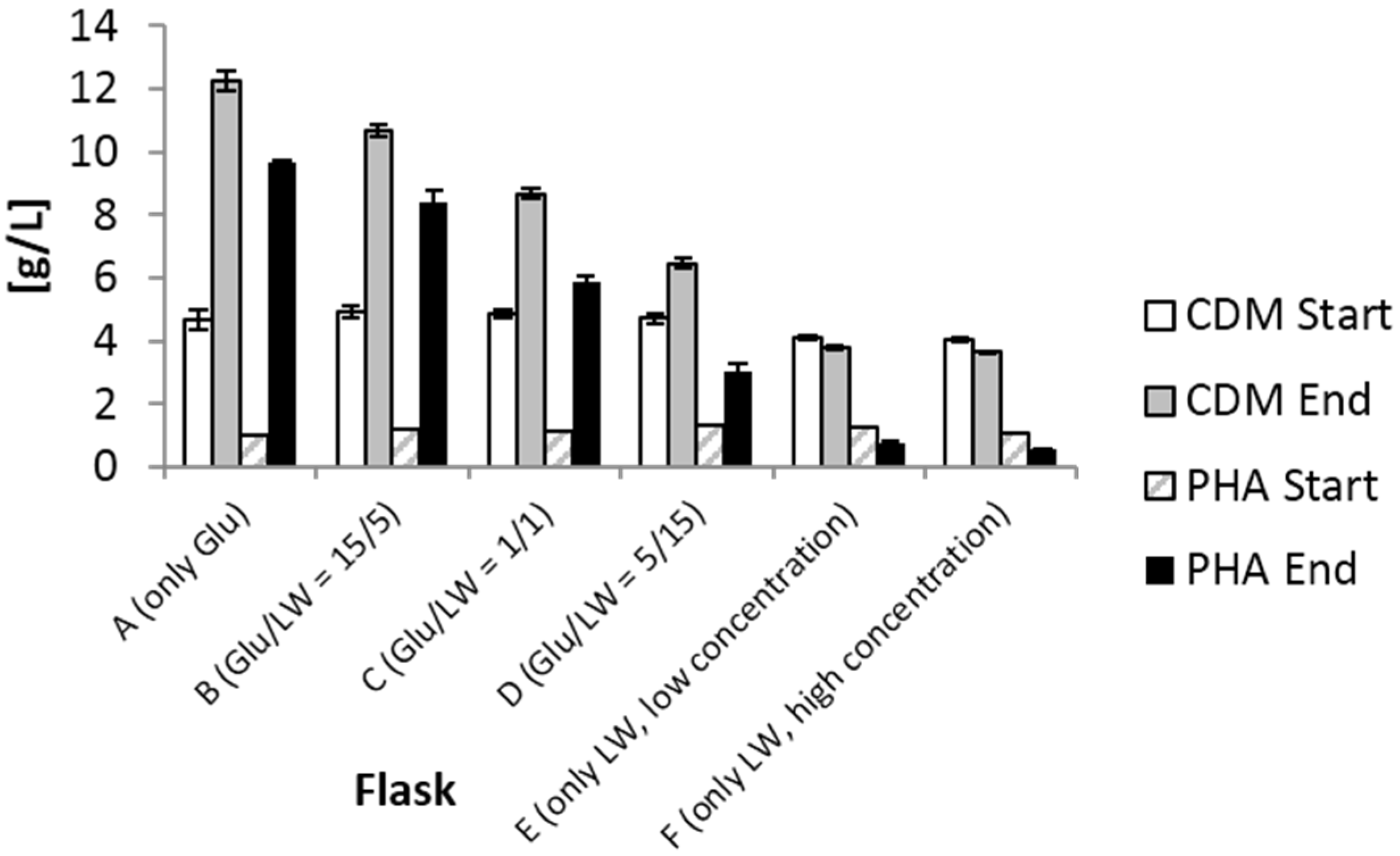
| Set-up | Carbon Feed (GLU/LW) | 3HV/PHA (%) | Mn × 105 (g/mol) | Mw × 105 (g/mol) | PDI | Tg (°C) | Tc (°C) | Tm (°C) |
|---|---|---|---|---|---|---|---|---|
| A | 20/0 | 0.0 | 5.08 | 7.22 | 1.30 | 7.7 | 56.5 | 181.2 |
| B | 15/5 | 0.2 | 4.83 | 6.36 | 1.32 | 6.2 | 53.9 | 180.0 |
| C | 10/10 | 0.6 | 4.27 | 5.74 | 1.34 | 6.6 | 54.4 | 179.1 |
| D | 5/15 | 0.0 | 3.49 | 4.89 | 1.40 | 5.6 | 49.9 | 178.7 |
| E | 0/5 | 0.0 | 3.44 | 4.85 | 1.41 | 6.9 | 49.6 | 177.7 |
| F | 0/10 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
2.2. Bioreactor Fermentation
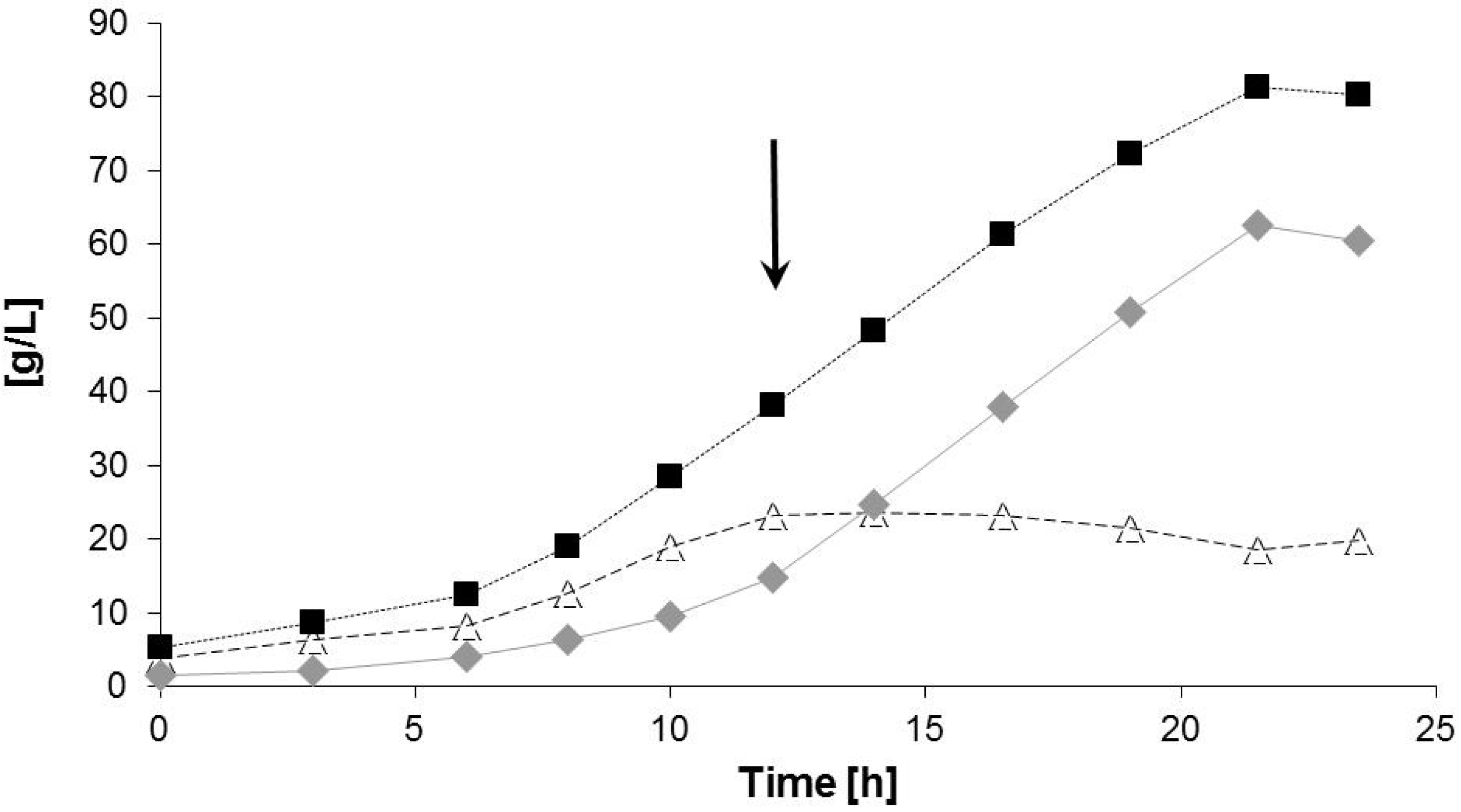
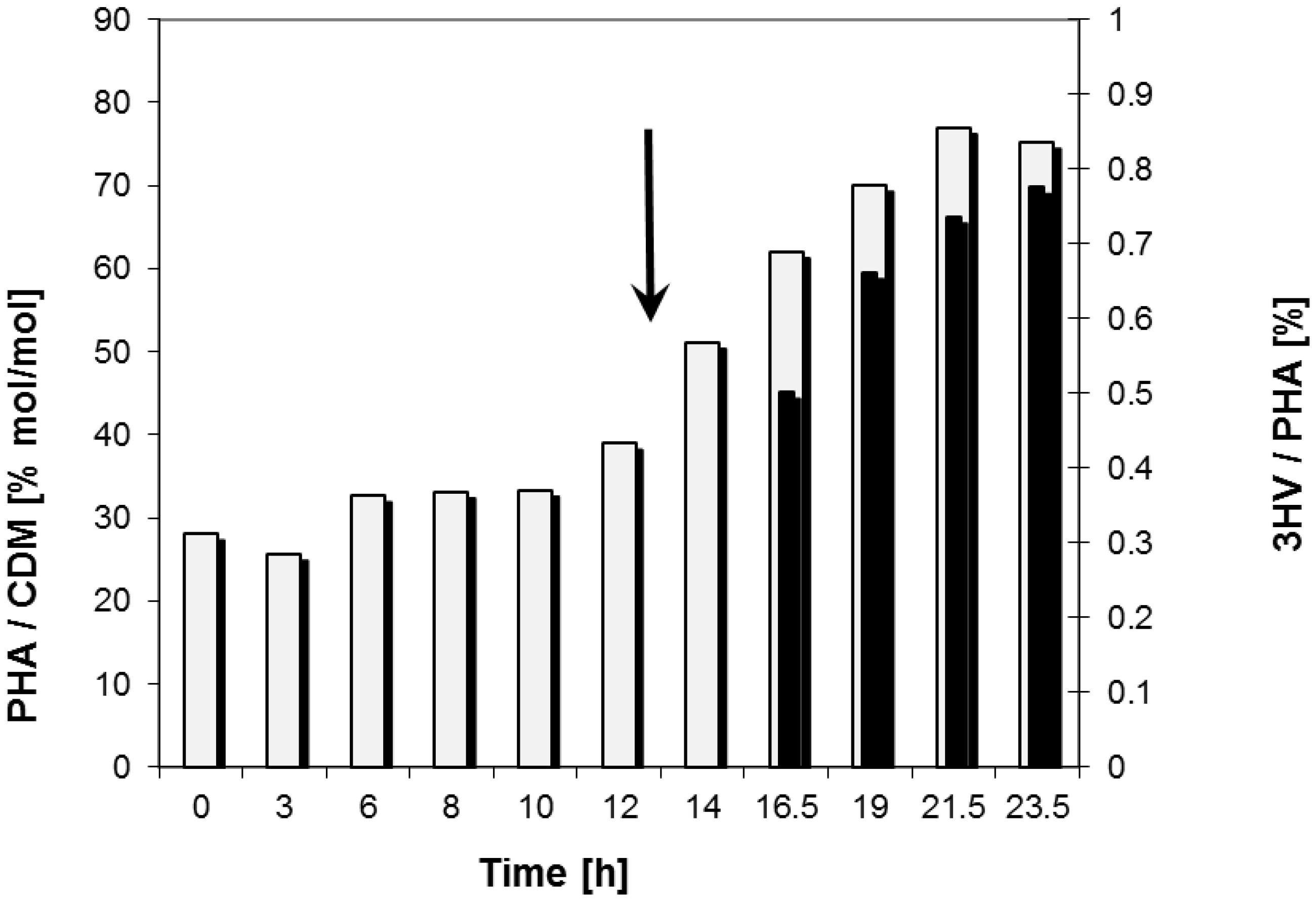
| Data for Bioprocess | Time Period (h) | |
|---|---|---|
| Max. cell dry mass (CDM) | 21.5 | 80.3 (g/L) |
| Max. PHA concentration | 21.5 | 60.5 (g/L) |
| Max. PHA content in cell mass | 21.5 | 77.0 (% (w/w)) |
| Max. 3HV content in PHA | 23.5 | 0.8 (% (w/w)) |
| Volumetric productivity for PHA | 0–23.5 | 2.84 (g/(L h)) |
| Specific volumetric productivity | 12–21.5 | 0.14 (g/(g h)) |
| Maximum specific growth rate µmax. | 6–12 | 0.20 (1/h) |
| Data for Polymer Characterization | ||
| Number average molar mass Mn × 105 | 23.5 | 3.52 (g/mol) |
| Weight average molar mass Mw × 105 | 23.5 | 5.39 (g/mol) |
| Polydispersity index (ĐM = Mw/Mn) | 23.5 | 1.53 |
| Melting point (Tm) | 23.5 | 176.3 (°C) |
| Melting enthalpy (δHm) | 23.5 | 90.7 (J/g) |
| Degree of crystallnity | 23.5 | 62.1 (%) |
| Cold crystallization (Tc) | 23.5 | 54.7 (°C) |
| Glass transition temperature (Tg) | 23.5 | 5.9 (°C) |
3. Discussion
4. Experimental Section
4.1. Production of Liquified Wood (LW)
4.2. Production Strain
4.3. Cultivation Conditions and Shake Flask Experiment
4.3.1. Strain Maintenance
4.3.2. Shake Flask Experiment
| Experimental Set-Up | GLU (g/L) | (LW) (g/L) * |
|---|---|---|
| A | 20 | 0 |
| B | 15 | 5 |
| C | 10 | 10 |
| D | 5 | 15 |
| E | 0 | 5 |
| F | 0 | 10 |
4.4. Bioreactor Experiment
4.5. Determination of Cell Dry Mass (CDM), Glucose and Ammonia
4.6. PHA Analysis
4.7. PHA Recovery
4.8. Determination of Melting Temperature, Glass Transition Temperature and Crystallinity
4.9. PHA Molar Mass Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gandini, A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar]
- Kržan, A.; Hemjinda, S.; Miertus, S.; Corti, A.; Chiellini, E. Standardization and Certification in the Area of Environmentally Degradable Plastics. Polym. Degrad. Stab. 2006, 91, 2819–2833. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Muhr, A.; Reiterer, A.; Braunegg, G. Polyhydroxyalkanoates: Biodegradable Polymeric Materials from Renewable Resources. Mater. Tehnol. 2013, 47, 5–12. [Google Scholar]
- Chen, G.Q. Plastics completely synthesized by bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria; Springer: Berlin, Germany; Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar]
- Khosravi-Darani, K.; Mokhtari, Z.B.; Amai, T.; Tanaka, K. Microbial production of poly (hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar]
- Koller, M.; Gasser, I.; Schmid, F.; Berg, G. Linking ecology with economy: Insights into polyhydroxyalkanoate-producing microorganisms. Eng. Life Sci. 2011, 11, 222–237. [Google Scholar] [CrossRef]
- Ayub, N.D.; Tribelli, P.M.; Lopez, N.I. Polyhydroxyalkanoates are essential for maintenance of redox state in the Antarctic bacterium Pseudomonas sp. 14-3 during low temperature adaptation. Extremophiles 2009, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Lenz, R.W. Polyesters from microorganisms. Adv. Biochem. Eng. Biotechnol. 2001, 71, 51–79. [Google Scholar] [PubMed]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Kovalcik, A.; Machovsky, M.; Kozakova, Z.; Koller, M. Designing packaging materials with viscoelastic and gas barrier properties by optimized processing of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with lignin. React. Funct. Polym. 2015, 94, 25–34. [Google Scholar] [CrossRef]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Adamus, G.; Sobota, M.; Musiol, M.; Janeczek, H.; Sikorska, W.; Kržan, A.; Žagar, E.; et al. Environmental degradation of blends of atactic poly [(R,S)-3-hydroxybutyrate] with natural PHBV in Baltic Sea water and compost with activated sludge. J. Polym. Environ. 2008, 16, 183–191. [Google Scholar] [CrossRef]
- Pietrini, M.; Roes, L.; Patel, M.K.; Chiellini, E. Comparative life cycle studies on poly (3-hydroxybutyrate)-based composites as potential replacement for conventional petrochemical plastics. Biomacromolecules 2007, 8, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, I.; Radecka, I.; Kowalczuk, M.; Adamus, G. Transesterification of PHA to Oligomers Covalently Bonded with (Bio) Active Compounds Containing Either Carboxyl or Hydroxyl Functionalities. PLoS ONE 2015, 10, e0120149. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyester. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar]
- Savenkova, L.; Gercberga, Z.; Bibers, I.; Kalnin, M. Effect of 3-hydroxy valerate content on some physical and mechanical properties of polyhydroxyalkanoates produced by Azotobacter chroococcum. Proc. Biochem. 2000, 36, 445–450. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Illieva, V.; Chiellini, E.; Braunegg, G. Novel precursors for production of 3-hydroxyvalerate-containing poly[(R)-hydroxyalkanoate]s. Biocat. Biotrans. 2014, 32, 161–167. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biot. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Kržan, A.; Žagar, E. Microwave driven wood liquefaction with glycols. Biores. Technol. 2009, 100, 3143–3146. [Google Scholar] [CrossRef] [PubMed]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N. Ultrasonically assisted liquefaction of lignocellulosic materials. Biores. Technol. 2012, 103, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kos, T.; Anžlovar, A.; Kunaver, M.; Huskić, M.; Žagar, E. Fast preparation of nanocrystalline cellulose by microwave-assisted hydrolysis. Cellulose 2014, 21, 2579–2585. [Google Scholar] [CrossRef]
- Keenan, T.M.; Tanenbaum, S.W.; Stipanovic, A.J.; Nakas, J.P. Production and characterization of Poly-β-hydroxyalkanoate copolymers from Burkholderia cepacia utilizing xylose and levulinic acid. Biotechnol. Prog. 2004, 20, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Rogers, P.L. Effect of levulinic acid on cell growth and poly-β-hydroxyalkanoate production by Alcaligenes sp. SH-69. Biotechnol. Lett. 1996, 18, 219–224. [Google Scholar] [CrossRef]
- Schmack, G.; Gorenflo, V.; Steinbüchel, A. Biotechnological production and characterization of polyesters containing 4-hydroxyvaleric acid and medium-chain-length hydroxyalkanoic acids. Macromolecules 1998, 31, 644–649. [Google Scholar] [CrossRef]
- Yu, J.; Stahl, H. Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Biores. Technol. 2008, 99, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, L.X.; Sato, S. Biopolyester synthesis and protein regulations in Ralstonia eutropha on levulinic acid and its derivatives from biomass refining. J. Biobased. Mat. Bioenerg. 2009, 3, 113–122. [Google Scholar] [CrossRef]
- Horvat, P.; Špoljarić, I.V.; Lopar, M.; Atlić, A.; Koller, M.; Braunegg, G. Mathematical modelling and process optimization of a continuous 5-stage bioreactor cascade for production of poly [-(R)-3-hydroxybutyrate] by Cupriavidus necator. Bioproc. Biosyst. Eng. 2012, 36, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Bona, R.; Hermann, C.; Horvat, P.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P.; Braunegg, G. Biotechnological production of poly(3-hydroxybutyrate) with Wautersia eutropha by application of green grass juice and silage juice as additional complex substrates. Biocatal. Biotransform. 2006, 23, 329–337. [Google Scholar] [CrossRef]
- Nonato, R.V.; Mantelatto, P.E.; Rossell, C.E.V. Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar]
- Ashby, R.D.; Solaiman, D.K.Y.; Strahan, G.D.; Zhu, C.; Tappan, R.C.; Nomura, C.T. Glycerine and levulinic acid: Renewable co-substrates for the fermentative synthesis of short-chain poly(hydroxyalkanoate) biopolymers. Biores. Technol. 2012, 118, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Holding, A.J.; Shewan, J.M. Bergey’s Manual of Determinative Bacteriology, 8th ed.; The Williams and Wilkins Company: Baltimore, MD, USA, 1974; p. 275. [Google Scholar]
- Rao, U.; Sridhar, R.; Sehgal, P.K. Biosynthesis and biocompatibility of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Küng, W. Wachstum und Poly-d-(-)-hydroxybuttersäure Akkumulation bei Alcaligenes latus. Ph.D. Thesis, Graz University of Technology, Graz, Austria, 1982. [Google Scholar]
- Koller, M.; Bona, R.; Chiellini, E.; Grillo Fernandes, E.; Horvat, P.; Kutschera, C.; Hesse, P.J.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Biores. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef] [PubMed]
- Braunegg, G.; Sonnleitner, B.; Lafferty, R.M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Koller, M. Novel Description of mcl-PHA Biosynthesis by Pseudomonas chlororaphis from Animal-Derived Waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Zinn, M. Tailor-made synthesis of polyhydroxyalkanoate. Eur. Cell Mat. 2003, 5, 38–39. [Google Scholar]
- Koller, M.; Chiellini, E.; Braunegg, G. Study on the Production and Re-use of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and Extracellular Polysaccharide by the Archaeon Haloferax mediterranei Strain DSM 1411. Chem. Biochem. Eng. Q. 2015, 29, 87–98. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, M.; Dias, M.M.d.S.; Rodríguez-Contreras, A.; Kunaver, M.; Žagar, E.; Kržan, A.; Braunegg, G. Liquefied Wood as Inexpensive Precursor-Feedstock for Bio-Mediated Incorporation of (R)-3-Hydroxyvalerate into Polyhydroxyalkanoates. Materials 2015, 8, 6543-6557. https://doi.org/10.3390/ma8095321
Koller M, Dias MMdS, Rodríguez-Contreras A, Kunaver M, Žagar E, Kržan A, Braunegg G. Liquefied Wood as Inexpensive Precursor-Feedstock for Bio-Mediated Incorporation of (R)-3-Hydroxyvalerate into Polyhydroxyalkanoates. Materials. 2015; 8(9):6543-6557. https://doi.org/10.3390/ma8095321
Chicago/Turabian StyleKoller, Martin, Miguel Miranda de Sousa Dias, Alejandra Rodríguez-Contreras, Matjaž Kunaver, Ema Žagar, Andrej Kržan, and Gerhart Braunegg. 2015. "Liquefied Wood as Inexpensive Precursor-Feedstock for Bio-Mediated Incorporation of (R)-3-Hydroxyvalerate into Polyhydroxyalkanoates" Materials 8, no. 9: 6543-6557. https://doi.org/10.3390/ma8095321