Valorization of Waste Obtained from Oil Extraction in Moringa Oleifera Seeds: Coagulation of Reactive Dyes in Textile Effluents
Abstract
:1. Introduction
2. Experimental Section
2.1. Moringa oleifera Oil Extraction
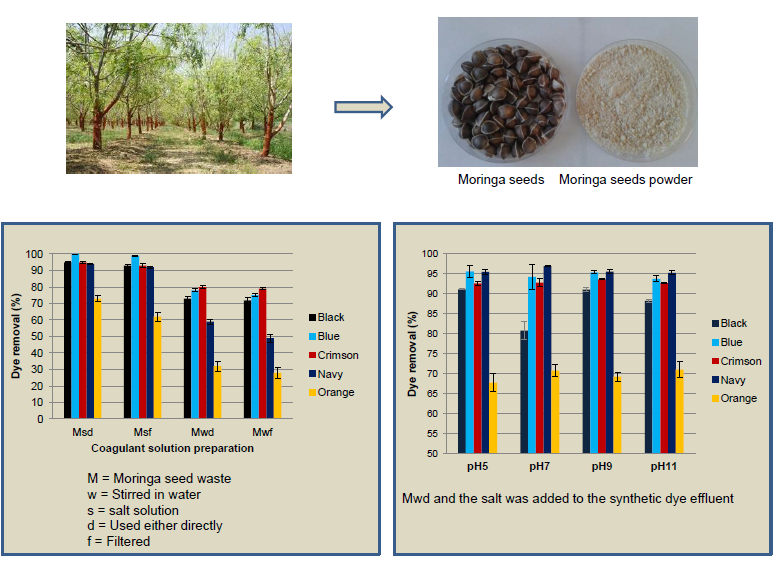
2.2. Preparation of Coagulant from Moringa oleifera
2.3. Preparation of Reactive Dye Solutions
2.4. Dye Removal Tests
2.5. Cotton Dyeing with Decolorized Water

3. Results and Discussion
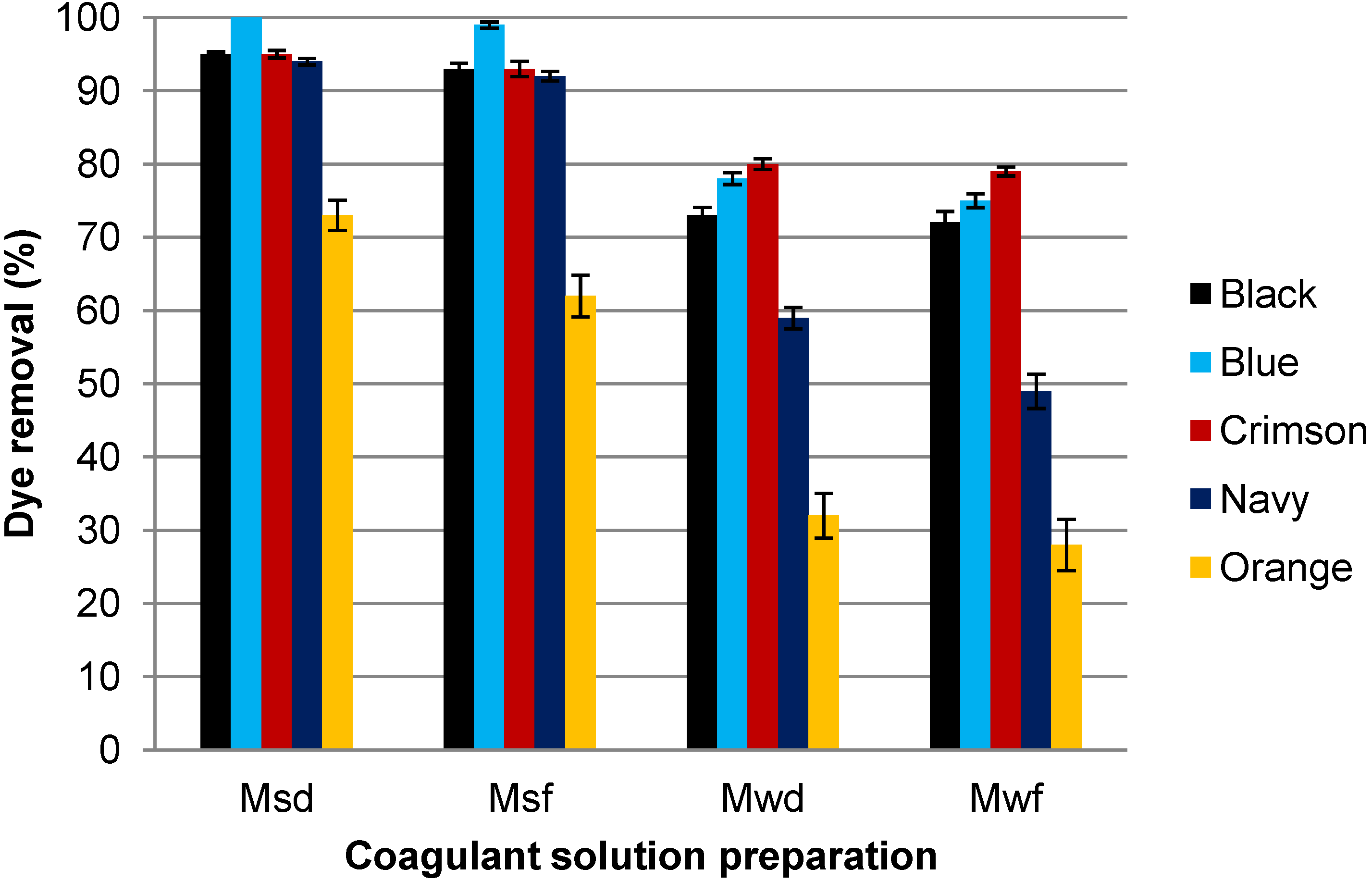
3.1. Influence of the Coagulant Solution Preparation
3.2. Influence of Dyeing Electrolyte
| Dye solution | Extraction | Black | Blue | Crimson | Navy | Orange |
|---|---|---|---|---|---|---|
| Dye removal (%) | ||||||
| Dye-w | Msf | 93 | 98 | 93 | 92 | 62 |
| Msd | 95 | 100 | 95 | 94 | 73 | |
| Dye-s | Mwf | 93 | 94 | 91 | 90 | 63 |
| Mwd | 95 | 97 | 93 | 95 | 68 | |
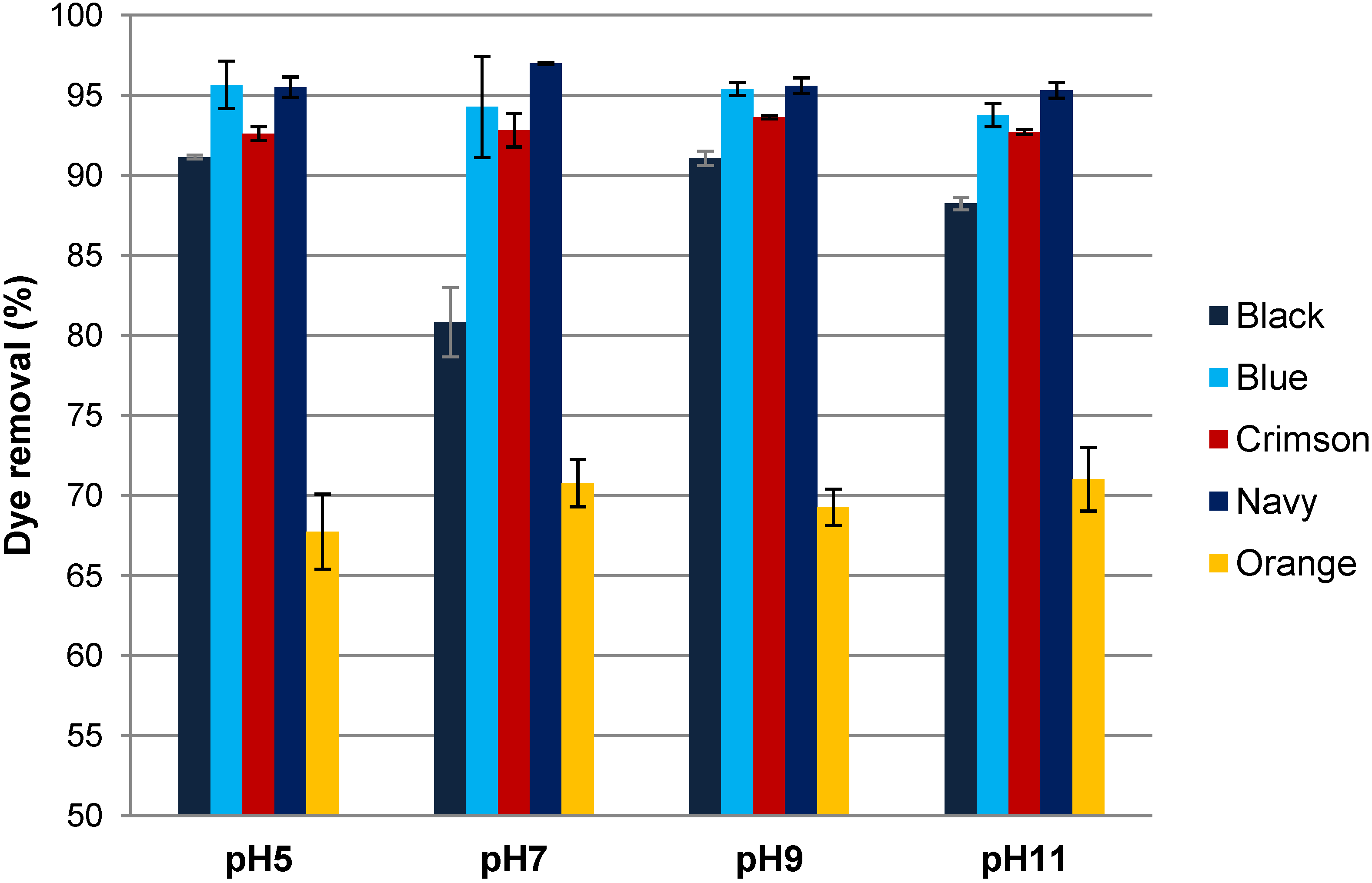
3.3. Influence of the pH
3.4. Influence of Coagulant Concentration

3.5. Efficiency of Moringa oleifera Waste versus Ferrous (III) Chloride
| Jar test | Black | Blue | Crimson | Navy | Orange | |
|---|---|---|---|---|---|---|
| Dye removal (%) | ||||||
| Moringa | V30 min | 83 | 90 | 89 | 96 | 54 |
| V24 h | 85 | 96 | 96 | 97 | 53 | |
| FeCl3 + poly | V30 min | 9 | 56 | 24 | 30 | 3 |
| V24 h | 4 | 53 | 20 | 25 | 0 | |
| Settled sludge volume (mL/L) | ||||||
| Moringa | V30 min | 38 | 150 | 100 | 130 | 33 |
| V24 h | 27 | 60 | 40 | 59 | 29 | |
3.6. Reuse of the Decolorized Effluent for New Dyeing Process
| Dyeing | DL | DC | DH | SL | SC | SH | DECMC(2:1) |
|---|---|---|---|---|---|---|---|
| Black | −1.35 | −0.59 | 0.27 | 0.67 | 1.79 | 1.18 | 1.09 |
| Blue | 0.22 | 0.13 | −0.34 | 0.96 | 4.04 | 2.45 | 0.18 |
| Crimson | 0.54 | −0.11 | −0.66 | 0.89 | 6.65 | 4.52 | 0.33 |
| Navy | −0.72 | 0.10 | −0.01 | 0.73 | 2.02 | 1.30 | 0.49 |
| Orange | 2.28 | −5.40 | −1.49 | 1.15 | 9.04 | 3.90 | 1.22 |
| Trichromie | 0.37 | −0.15 | 0.06 | 0.71 | 1.79 | 1.18 | 0.27 |
| Parameter | Black | Blue | Crimson | Navy | Orange |
|---|---|---|---|---|---|
| Dye Removal (%) | 85 | 96 | 96 | 97 | 53 |
| TOC (mg/L) | 101.1 | 95.9 | 91.4 | 97.4 | 108.0 |
| DECMC(2:1) | 1.09 | 0.18 | 0.33 | 0.49 | 1.22 |
4. Conclusions
Supplementary Information
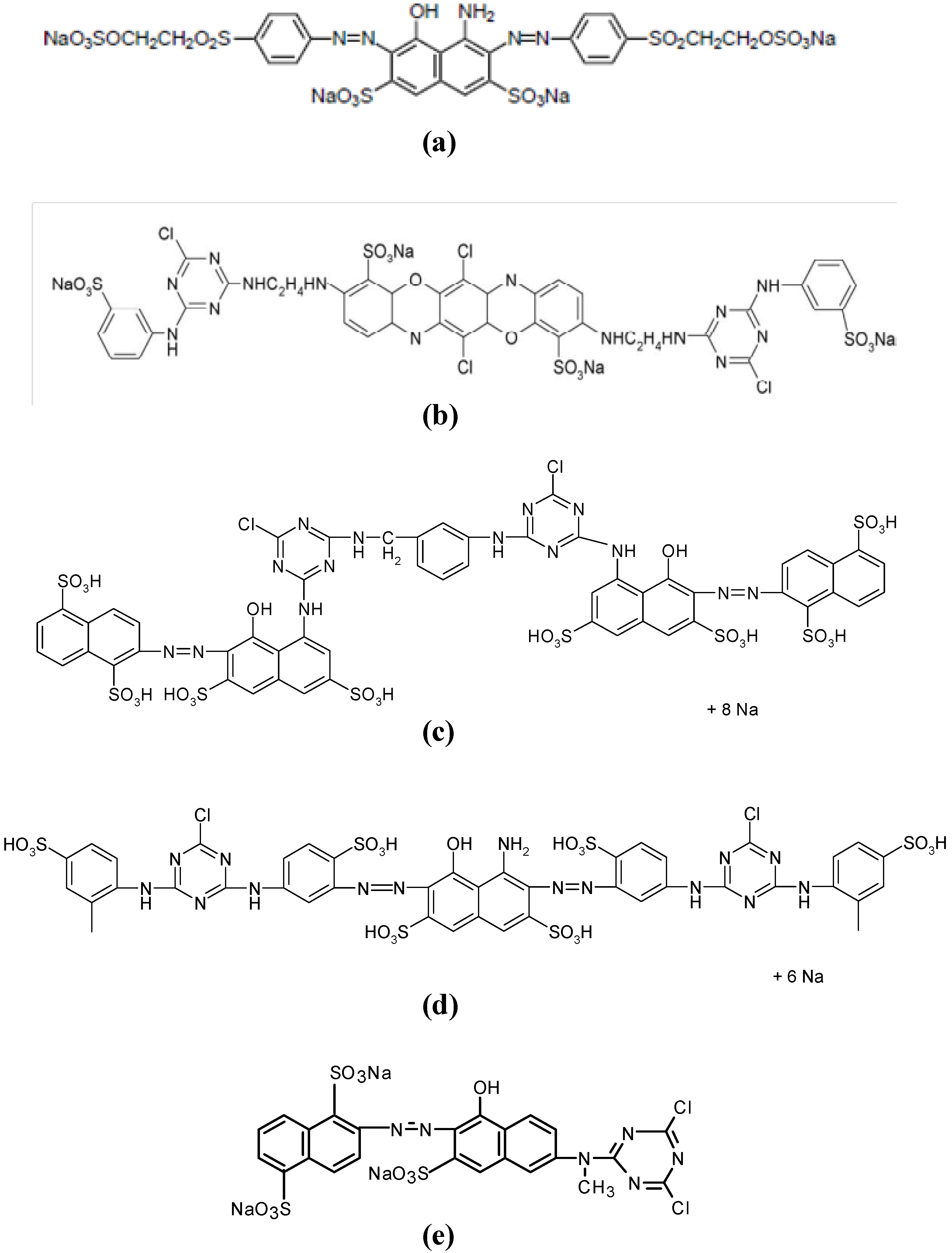
| Commercial Name | C.I. Name | Chromophore/reactive group | Number of sulfonic groups | λ max (nm) | Chemical structure (Figure 1) |
|---|---|---|---|---|---|
| Remazol Black B-133 | Reactive Black 5 | Bis-azo/bis-vinyl sulfone | 4 | 599 | a |
| Procion Blue H-EXL | Reactive Blue 198 | Bis-oxazine/bis-monochlorotriazine | 4 | 625 | b |
| Procion Crimson H-EXL | Reactive Red 231 | Bis-azo/bis-monochlorotriazine | 8 | 546 | c |
| Procion Navy H-EXL | Not available | Bis-azo/bis-monochlorotriazine | 8 or 6 | 606 | c and d (mixture) |
| Procion Orange MX-2R | Reactive Orange 4 | Azo/dichlorotriazine | 3 | 490 | e |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mughal, M.H.; Ali, G.; Srivastava, P.S.; Iqbal, M. Improvement of drumstick (Moringa pterygosperms Gaetn.)—A unique source of food aand medicine through tissue culture. Hamdard Med. 1999, 42, 37–42. [Google Scholar]
- Oliveira, J.T.A.; Silveira, S.B.; Vasconcelos, K.M.; Cavada, B.S.; Moreira, R.A. Compositional and nutritional attributes of sedes from the multiple purpose tree Moringa oleifera Lamarck. J. Sci. Food Agric. 1999, 79, 815–820. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Bhanger, M.I. Analytical characterization of Moringa oleífera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem. 2003, 51, 6558–6563. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.; Anwar, F.; Moser, B.R.; Knothe, G. Moringa Oleifera oil: A possible source of biodiesel. Bioresour. Technol. 2008, 99, 8175–8179. [Google Scholar] [CrossRef] [PubMed]
- Ndabigengesere, A.; Narasiah, K.S. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.S.; O’neill, J.G. A study of the parameters affecting the effectiveness of Moriga oleífera in drinking water purification. Phys. Chem. Earth 2010, 35, 791–797. [Google Scholar] [CrossRef]
- Yin, C.Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1449. [Google Scholar] [CrossRef]
- Bhuptawat, H.; Folkard, G.K.; Chaudhari, S. Innovative physico-chemical treatment of wastewater incorporating Moringa Oleifera seed coagulant. J. Hazard. Mater. 2007, 142, 477–482. [Google Scholar] [CrossRef] [PubMed]
- McConnachie, G.L.; Folkard, G.K.; Mtawali, M.A.; Sutherland, J.P. Field trials of appropriate hydraulic flocculation processes. Water Res. 1999, 33, 1425–1434. [Google Scholar] [CrossRef]
- Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.S.; O’neill, J.G.A. A comparison between Moringa oleifera and chemical coagulants in the purification of drinking water—An alternative sustainable solution for developing countries. Phys. Chem. Earth 2010, 35, 798–805. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.D.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Kyzas, G.Z. A decolorization technique with spent “Greek Coffee” grounds as zero-cost adsorbents for industrial textile wastewaters. Materials 2012, 5, 2069–2087. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Matis, K.A. The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials 2013, 6, 5131–5158. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef]
- Carvalho, G.; Delée, W.; Novais, J.M.; Pinheiro, H.M. A factorially-designed study of physicochemical reactive dye colour removal from simulated cotton textile processing wastewaters. Color. Technol. 2002, 118, 215–219. [Google Scholar] [CrossRef]
- Golob, V.; Vinder, A.; Simonic, M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dyes Pigm. 2005, 67, 93–97. [Google Scholar] [CrossRef]
- Shende, R.V.; Mahajani, V.V. Wet oxidative regeneration of activated carbon loaded with reactive dye. Waste Manag. 2002, 22, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Liu, X.; Bo, L.; Chen, S.; Zhao, Y.; Cui, X. Regeneration of acid orange-7 exhausted granular activated carbons with microwave irradiation. Water Res. 2004, 38, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Oo, M.H.; Kekre, K.A. Nanofiltration for recovering wastewater from a specific dyeing facility. Sep. Purif. Technol. 2007, 56, 199–203. [Google Scholar] [CrossRef]
- Petrinic, I.; Andersen, N.P.; Sostar-Turk, S.; le Marechal, A.M. The removal of reactive dye printing compounds using nanofiltration. Dyes Pigm. 2007, 74, 512–518. [Google Scholar] [CrossRef]
- Sun, S.P.; Hatton, T.A.; Chan, S.Y.; Chung, T.S. Novel thin-film composite nanofiltration hollow fiber membranes with double repulsion for effective removal of emerging organic matters from water. J. Membr. Sci. 2012, 401–402, 152–162. [Google Scholar]
- Ong, Y.K.; Li, F.Y.; Sun, S.P.; Zhao, B.W.; Liang, C.Z.; Chung, T.S. Nanofiltration hollow fiber membranes for textile wastewater treatment: Lab-scale and pilot-scale studies. Chem. Eng. Sci. 2014, 114, 51–57. [Google Scholar] [CrossRef]
- De Faria, L.A.; Santana, M.H.P.; da Silva, L.M.; Freitas, A.C.; Boodts, J.F.C.; Fernandes, K.C. Application of electrochemically generated ozone to the discoloration and degradation of solutions containing the dye Reactive Orange 112. J. Hazard. Mater. 2009, 164, 10–17. [Google Scholar]
- Wu, J.; Doan, H.; Upreti, S. Decolorization of aqueous textile reactive dye by ozone. Chem. Eng. J. 2008, 142, 156–160. [Google Scholar] [CrossRef]
- Papic, S.; Vujevic, D.; Koprivanac, N.; Sinko, D. Decolourization and mineralization of commercial reactive dyes by using homogeneous and heterogeneous Fenton and UV/Fenton processes. J. Hazard. Mater. 2009, 164, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, T.; Lu, X.; Wang, J.; Wong, F.-S. Rapid decolorization and mineralization of simulated textile wastewater in a heterogeneous Fenton like system with/without external energy. J. Hazard. Mater. 2009, 165, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of Reactive Orange 4 by TiO2-UV process. Dyes Pigm. 2006, 68, 133–142. [Google Scholar] [CrossRef]
- Visa, T.; Sanchez, M.; López-Grimau, V.; Navarro, R.; Reche, S.; Gutiérrez-Bouzán, M.C. Photocatalysis with titanium dioxide to remove colour of exhausted reactive dyebaths without pH modification. Desalin. Water Treat. 2012, 45, 91–99. [Google Scholar] [CrossRef]
- Lopez-Grimau, V.; Gutierrez, M.C. Decolourisation of simulated reactive dyebath effluents by electrochemical oxidation assisted by UV light. Chemosphere 2006, 62, 106–112. [Google Scholar]
- Morsi, M.S.; Al-Sarawy, A.A.; Shehab El-Dein, W.A. Electrochemical degradation of some organic dyes by electrochemical oxidation on a Pb/PbO2 electrode. Desalin. Water Treat. 2011, 26, 301–308. [Google Scholar] [CrossRef]
- Liang, C.Z.; Sun, S.P.; Li, F.Y.; Ong, Y.K.; Chung, T.S. Treatment of highly concentrated wastewater containing multiple syntethic dyes by a combined process of coagulation/flocculation and nanofiltration. J. Membr. Sci. 2014, 469, 306–315. [Google Scholar] [CrossRef]
- Chemical Insight & Forecasting: IHS Chemical. Chemical Economics Handbook: Dyes. Available online: http://www.ihs.com/products/chemical/planning/ceh/dyes.aspx (accessed on 12 March 2014).
- Solis, M.; Solis, A.; Perez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar]
- International Agency for Research on Cancer (IARC). Overall Evaluations of Carcinogenicity to Humans. In IARC Monographs; IARC: Lyon, France, 2004. [Google Scholar]
- López-Grimau, V.; Riera-Torres, M.; Lopez-Mesas, M.; Gutierrez-Bouzan, C. Removal of aromatic amines and decolourisation of azo dye baths by electrochemical treatment. Color. Technol. 2013, 129, 267–273. [Google Scholar]
- Carneiro, P.A.; Osugi, M.E.; Fugivara, C.S.; Boralle, N.; Furlan, M.; Zanoni, M.V. Evaluation of different electrochemical methods on the oxidation and degradation of Reactive Blue 4 in aqueous solution. Chemosphere 2005, 59, 431–439. [Google Scholar] [CrossRef] [PubMed]
- López-Grimau, V.; Gutiérrez-Bouzán, C. Selection of decolorization methods of reactive dye baths for reuse purposes. In Dyeing: Processes, Techniques and Applications; Fu, J., Ed.; Nova Publishers Inc.: New York, NY, USA, 2013; Chapter 10; pp. 205–216. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.M.; Liang, T.M. A feasibility study of industrial wastewater recovery using electrodialysis reversal. Desalination 2008, 221, 433–439. [Google Scholar] [CrossRef]
- Beltran-Heredia, J.; Sanchez-Martin, J. Azo dye removal by Moringa oleífera seed extract coagulant. Color. Technol. 2008, 124, 310–317. [Google Scholar]
- Beltran-Heredia, J.; Sanchez-Martin, J.; Delagado-Regalado, A.; Jurado-Bustos, C. Removal of Alizarin Violet 3R (anthraquinonic dye) from aqueous solutions by natural coagulants. J. Hazard. Mater. 2009, 170, 43–50. [Google Scholar]
- Ortiz-Palafox, J.; Navarrete, A.; Sacramento-Rivero, J.C.; Rubio-Atocha, C.; Acereto-Escoffie, P.; Rocha-Uribe, J.A. Extraction and characterization of oil from Moringa Oleifera using supercritical CO2 and traditional solvents. Am. J. Anal. Chem. 2012, 3, 946–949. [Google Scholar]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic graphene oxide: Effect of preparation route on Reactive Black 5 adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef]
- European Commission. Integrated Pollution Prevention and Control (IPPC). Reference Document on Best Available Techniques for the Textile Industry. Published: 2003. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/txt_bref_0703.pdf (accessed on 18 August 2014).
- Lacasse, K.; Baumann, W. Textile Chemicals. Environmental Data and Facts, 1st ed.; Springer-Verlag: Heidelberg, Germany, 2004. [Google Scholar]
- DyStar, Inc. Work Procedures. Industrial Washing Procedure, Excel Washing; DyStar, Inc.: Frankfurt, Germany, 2004. [Google Scholar]
- AENOR (Spanish Association for Standardization and Certification). Textiles. Ensayos de solidez del Color: Cálculo de las Diferencias de Color; UNE-EN ISO105-J03: 1997; Asociación Española de Normalización y Certificación: Madrid, Spain, 1997. (In Spanish) [Google Scholar]
- Christie, R.M. Environmental Aspects of Textile Dyeing, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2007. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vilaseca, M.; López-Grimau, V.; Gutiérrez-Bouzán, C. Valorization of Waste Obtained from Oil Extraction in Moringa Oleifera Seeds: Coagulation of Reactive Dyes in Textile Effluents. Materials 2014, 7, 6569-6584. https://doi.org/10.3390/ma7096569
Vilaseca M, López-Grimau V, Gutiérrez-Bouzán C. Valorization of Waste Obtained from Oil Extraction in Moringa Oleifera Seeds: Coagulation of Reactive Dyes in Textile Effluents. Materials. 2014; 7(9):6569-6584. https://doi.org/10.3390/ma7096569
Chicago/Turabian StyleVilaseca, Mercè, Víctor López-Grimau, and Carmen Gutiérrez-Bouzán. 2014. "Valorization of Waste Obtained from Oil Extraction in Moringa Oleifera Seeds: Coagulation of Reactive Dyes in Textile Effluents" Materials 7, no. 9: 6569-6584. https://doi.org/10.3390/ma7096569