Life Cycle Assessment of Biochar versus Metal Catalysts Used in Syngas Cleaning
Abstract
:1. Introduction
1.1. Life Cycle Assessment of Biochar Production and Use
1.2. Variability and Uncertainty in Life Cycle Assessment Studies
2. Methodology
2.1. Functional Unit
2.2. System Boundaries
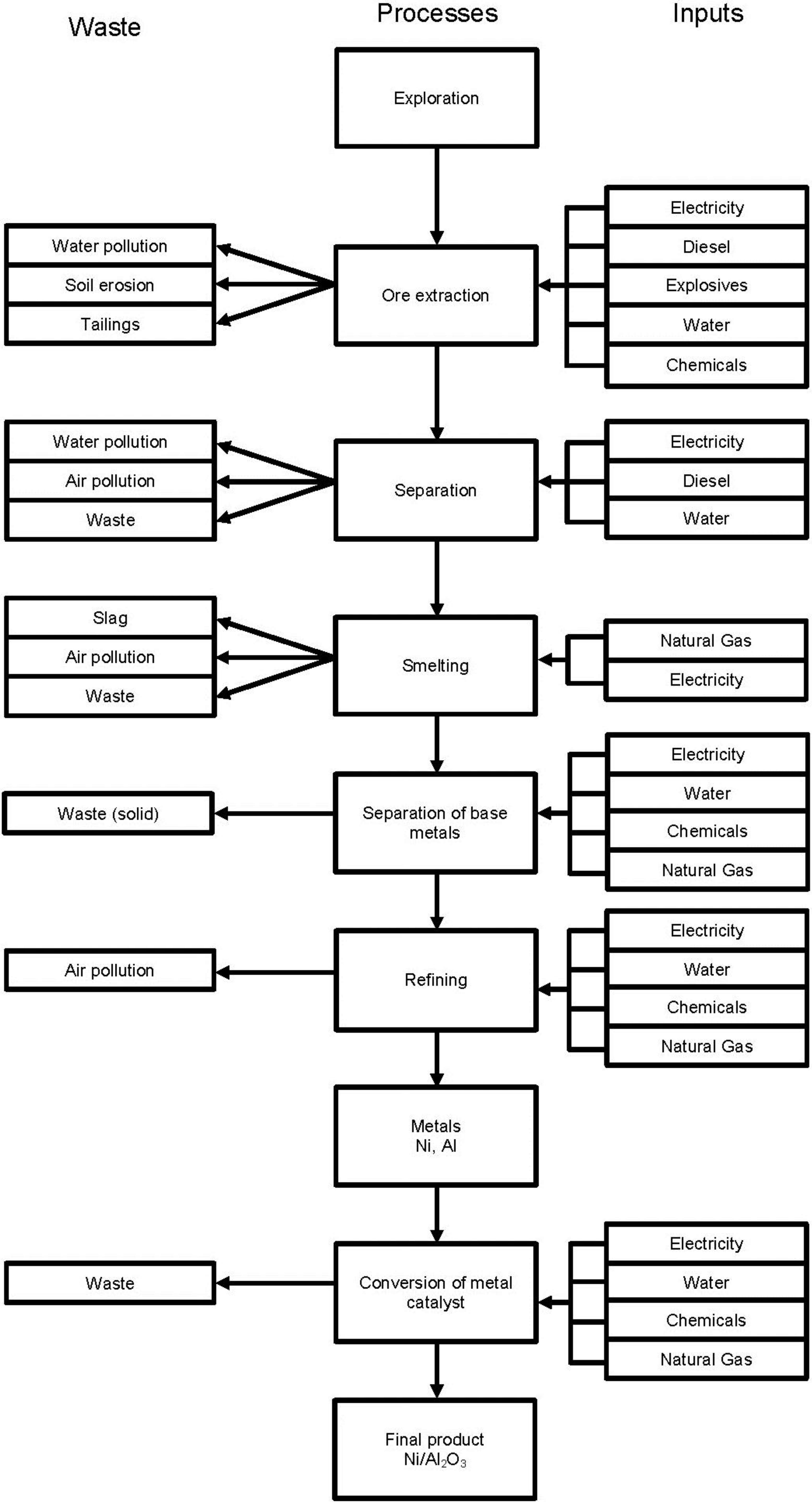
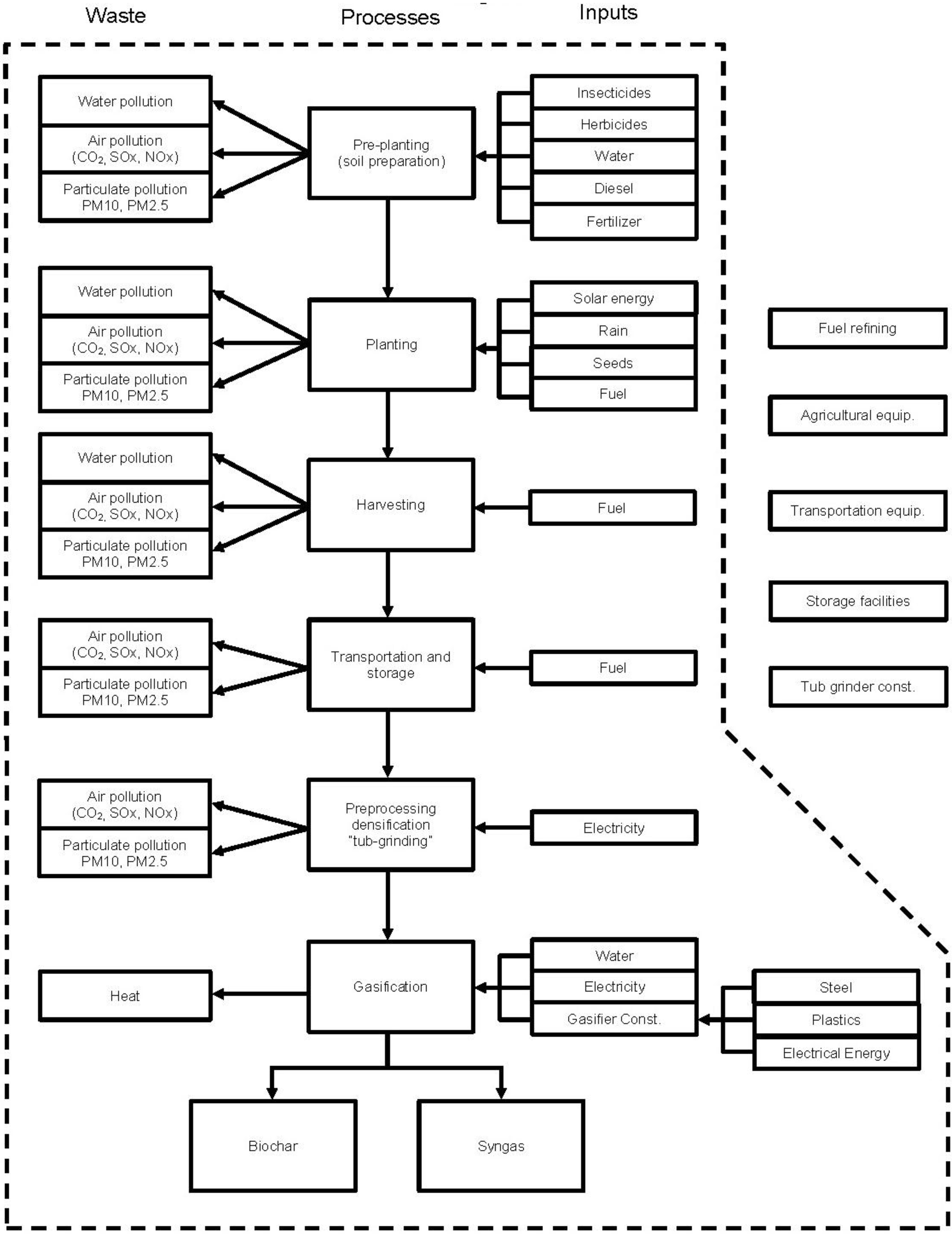
2.3. Assumptions
2.4. Assessment Tool and Method
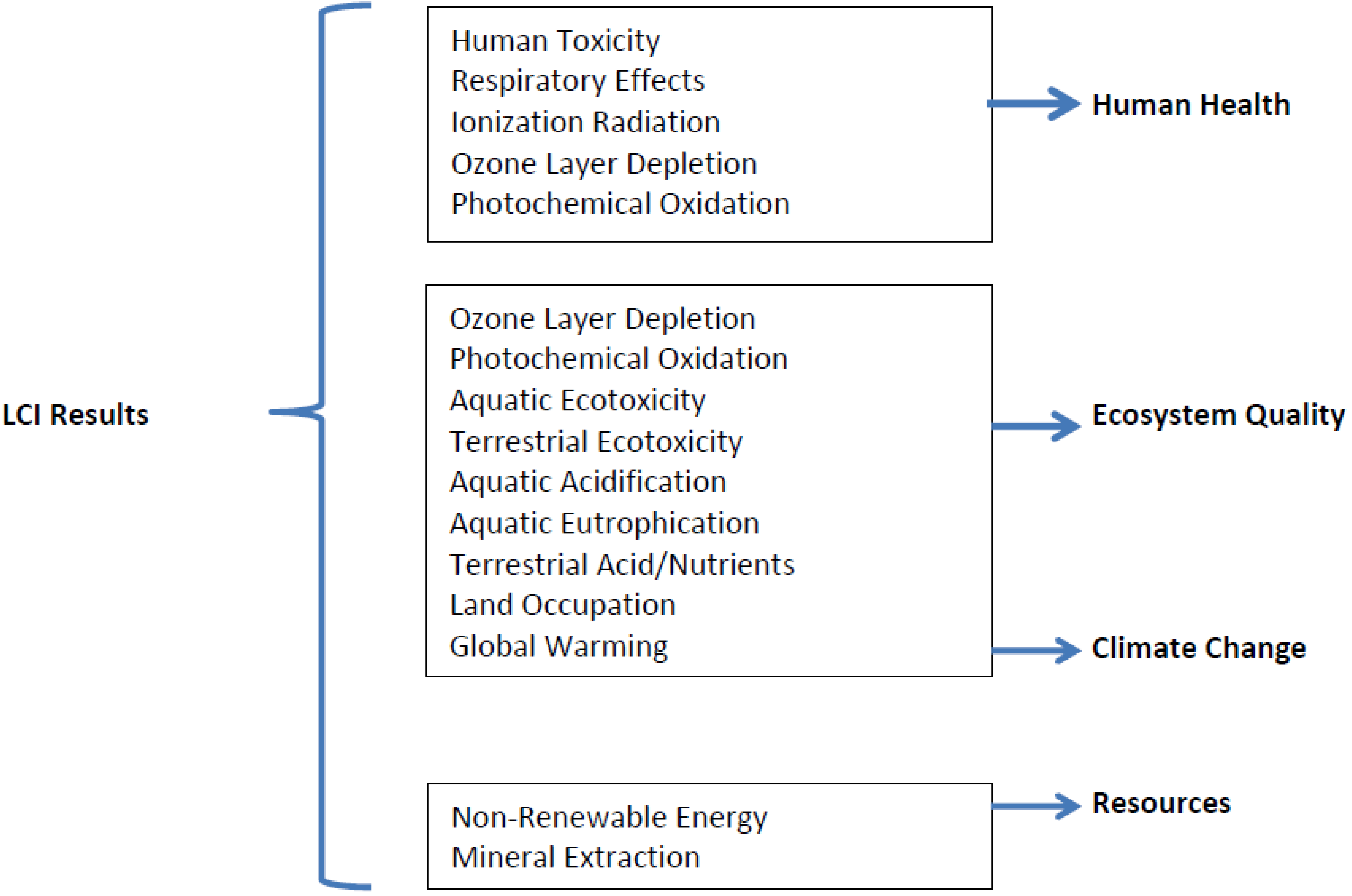
2.5. Life Cycle Inventory
2.6. Metal Catalyst Inventory
| Category | Unit process | Quantity |
|---|---|---|
| Resource (input) | Coal, in ground | 3.1 kg |
| Iron (Fe, ore) | 7.4 × 10−4 kg | |
| Limestone (CaCO3) | 0.4 kg | |
| Natural gas, in ground | 3.5 kg | |
| Ni, in ground | 2.5 kg | |
| Oil, in ground | 4.5 kg | |
| Uranium (U, ore) | 2.5 × 10−5 kg | |
| Total water used | 309 L | |
| Technosphere (input) | Total primary energy | 455 MJ |
| Emission to air (output) | Carbon dioxide | 26,337 g |
| Carbon monoxide | 62 g | |
| Nitrogen oxides, NO2 | 85 g | |
| Nitrous oxide | 2.0 g | |
| Emission to air (output) | Particulates | 74 g |
| Sulfur oxides, SO2 | 2,205 g | |
| Methane | 47 g | |
| Hydrocarbons | 22 g | |
| Ni | 6.1 g | |
| Chromium | 3.3 × 10−3 g | |
| Arsenic | 1.0 g | |
| Copper | 1.2 g | |
| Cobalt | 5.6 × 10−2 g | |
| Zinc | 0.19 g | |
| Lead | 0.53 g | |
| Cadmium | 3.7 × 10−3 g | |
| Mercury | 3.6 × 10−2 g | |
| Silver | 1.1 g | |
| Metals | 0.23 g | |
| Ammonia | 4.7 g | |
| Chloride | 1.3 × 10−3 g | |
| Dioxins | 4.4 × 10−7 g | |
| Volatile organic compounds | 2.7 g | |
| Hydrogen chloride | 0.98 g | |
| Hydrogen cyanide | 3.9 × 10−5 g | |
| Hydrogen fluoride | 5.9 × 10−2 g | |
| Hydrogen sulfide | 4.6 × 10−2 g | |
| Sulfuric acid | 40 g | |
| Emission to water (output) | Biochemical oxygen demand | 1.1 g |
| Chemical oxygen demand | 8.7 g | |
| Sulfates | 186 g | |
| Nitrogenous matter, as N | 269 g | |
| Phosphates, as P | 9.9 × 10−3 g | |
| Total organic compounds | 0.43 g | |
| Arsenic | 6.0 × 10−4 g | |
| Ni | 0.14 g | |
| Copper | 8.7 × 10−3 g | |
| Zinc | 1.3 × 10−3 g | |
| Lead | 4.1 × 10−2 g | |
| Mercury | 4.0 × 10−5 g | |
| Silver | 1.8 × 10−4 g | |
| Cadmium | 4.2 × 10−5 g | |
| Chromium | 3.3 × 10−4 g | |
| Acids | 1.4 × 10−2 g | |
| Emission to soil (output) | Waste rock and backfill | 175 kg |
| Tailing and other process residues | 187 kg | |
| Other solid materials | 1.8 kg |
2.7. Biochar Catalyst Inventory
| Category | Unit process | Quantity |
|---|---|---|
| Resource (input) | Carbon dioxide | 1.5 × 103 kg |
| Energy, from biomass | 1.5 × 104 MJ | |
| Occupation, pasture and meadow | 0.68 ha | |
| Transformation from permanent crop | 2.25 × 10−2 ha | |
| Transformation from pasture and meadow | 2.25 × 10−2 ha | |
| Transformation from arable | 2.25 × 10−2 ha | |
| Technosphere * (input) | Tillage, rotary cultivator and rolling | 6.8 × 10−3 ha |
| Fertilizer | 0.068 ha | |
| Planting | 0.068 ha | |
| Mowing, by rotary mower | 9.33 × 10−2 ha | |
| Baling | 9.33 × 10−2 ha | |
| Dried roughage store, non-ventilated | 9.57 × 10−8 m3 | |
| Conveyor belt, at plant | 3.47 × 10−5 m | |
| Fodder loading, by self-loading trailer | 2.2654 m3 | |
| Maize drying | 50 kg | |
| Grinding | 0.97 tn.sh | |
| Loading bales | 1.43 p | |
| Agricultural machinery | 0.9 kg | |
| Electricity, at grid | 63.93 kW·h | |
| Transport, tractor and trailer | 7.42 tkm | |
| Transport, combination truck | 182.6 tkm | |
| Transport, Train | 200 tkm | |
| Transport, Barge | 11.3 tkm | |
| Emission to air (output) | Carbon dioxide, biogenic | 295 kg |
| Water | 333 kg |
2.8. Allocation Method
2.9. Sensitivity Analysis
3. Results and Discussion
| Impact category | Unit | Total | NiO production (%) | Alumina production (%) | Mixing process (%) |
|---|---|---|---|---|---|
| Carcinogens | kg C2H3Cl(eq) | 3.51 × 103 | 92.9 | 5.1 | 1.32 |
| Non-carcinogens | kg C2H3Cl(eq) | 697 | 86.4 | 13.1 | 0.449 |
| Respiratory inorganics | kg PM2.5(eq) | 11.7 | 93.1 | 6.27 | 0.647 |
| Ionizing radiation | Bq C-14(eq) | 4.19 × 103 | 17 | 82.7 | 0.243 |
| Ozone layer depletion | kg CFC-11(eq) | 7.15 × 10−5 | 29.3 | 70.3 | 0.418 |
| Respiratory organics | kg C2H4(eq) | 2.59 | 88.4 | 10.3 | 1.24 |
| Aquatic ecotoxicity | kg TEG water | 1.37 × 106 | 88.7 | 10.2 | 1.11 |
| Terrestrial ecotoxicity | kg TEG soil | 1.87 × 105 | 93.4 | 6.56 | 0.0143 |
| Terrestrial acid/nutri | kg SO2(eq) | 167 | 91.7 | 7.67 | 0.598 |
| Land occupation | m2org.arable | 2.16 | 15.4 | 84.4 | 0.22 |
| Aquatic acidification | kg SO2(eq) | 144 | 95.3 | 4.08 | 0.665 |
| Aquatic eutrophication eutrophication | kg PO4(P-lim) | 0.14 | 62.8 | 36.4 | 0.835 |
| Global warming | kg CO2(eq) | 2.95 × 103 | 82.3 | 16.9 | 0.776 |
| Non-renewable energy | MJ primary | 1.73 × 105 | 90.9 | 7.91 | 1.19 |
| Mineral extraction | MJ surplus | 2.34 × 103 | 78 | 22 | 0.00821 |
3.1. Life Cycle Assessment of Nickel Catalyst Production
3.2. Life Cycle Assessment of Biochar Catalyst Production
| Impact category | Unit | Total | Switchgrass production (%) | Gasification process (%) |
|---|---|---|---|---|
| Carcinogens | kg C2H3Cl(eq) | 130 | 6.25 | 93.8 |
| Non-carcinogens | kg C2H3Cl(eq) | 12.4 | 33.1 | 66.9 |
| Respiratory inorganics | kg PM2.5(eq) | 0.344 | 41.5 | 58.5 |
| Ionizing radiation | Bq C-14(eq) | 283 | 73.5 | 26.5 |
| Ozone layer depletion | kg CFC-11(eq) | 4.85 × 10−6 | 80.7 | 19.3 |
| Respiratory organics | kg C2H4(eq) | 5.6 | 1.14 | 98.9 |
| Aquatic ecotoxicity | kg TEG water | 5.32 × 104 | 23.8 | 76.2 |
| Terrestrial ecotoxicity | kg TEG soil | 4820 | 96.7 | 3.3 |
| Terrestrial acid/nutri | kg SO2(eq) | 7.19 | 62.7 | 37.3 |
| Land occupation | m2org.arable | 8,300 | 100 | 5.84 × 10−4 |
| Aquatic acidification | kg SO2(eq) | 3.67 | 31 | 69 |
| Aquatic eutrophication | kg PO4(P-lim) | 8.89 × 10−3 | 59.3 | 40.7 |
| Global warming | kg CO2(eq) | 206 | 69.3 | 30.7 |
| Non-renewable energy | MJ primary | 7,550 | 27.6 | 72.4 |
| Mineral extraction | MJ surplus | 2.7 | 70.8 | 29.2 |
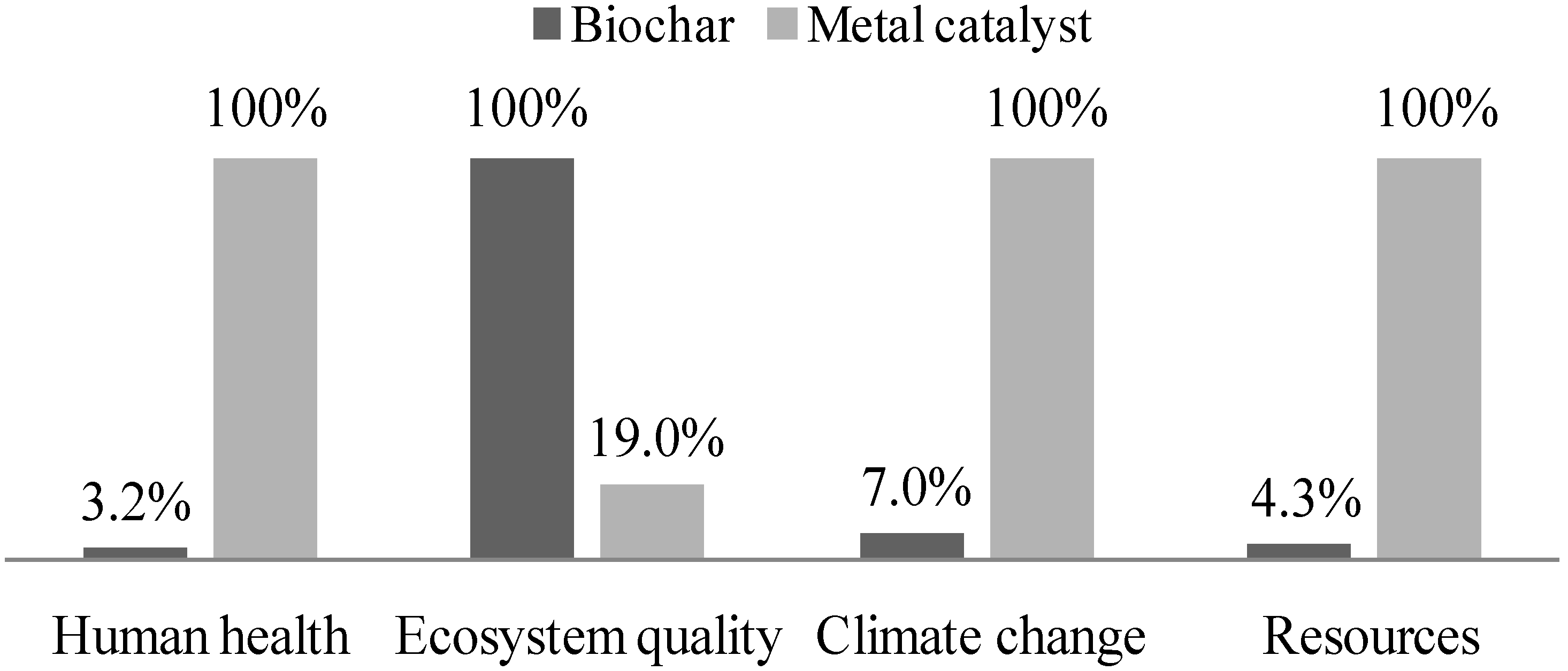
3.3. Comparison Analysis
| Impact category | Unit | Metal catalyst (396 kg) | Biochar catalyst (953 kg) |
|---|---|---|---|
| Carcinogens | kg C2H3Cl(eq) | 3.51 × 103 | 130 |
| Non-carcinogens | kg C2H3Cl(eq) | 697 | 12.4 |
| Respiratory inorganics | kg PM2.5(eq) | 11.7 | 0.344 |
| Ionizing radiation | Bq C-14(eq) | 4.19 × 103 | 283 |
| Ozone layer depletion | kg CFC-11(eq) | 7.15 × 10−5 | 4.85× 10−6 |
| Respiratory organics | kg C2H4(eq) | 2.59 | 5.6 |
| Aquatic ecotoxicity | kg TEG water | 1.37 × 106 | 5.32 × 104 |
| Terrestrial ecotoxicity | kg TEG soil | 1.87 × 105 | 4,820 |
| Terrestrial acid/nutri | kg SO2(eq) | 167 | 7.19 |
| Land occupation | m2org.arable | 2.16 | 8300 |
| Aquatic acidification | kg SO2(eq) | 144 | 3.67 |
| Aquatic eutrophication | kg PO4(P-lim) | 0.14 | 8.89 × 10−3 |
| Global warming | kg CO2(eq) | 2.95 × 103 | 206 |
| Non-renewable energy | MJ primary | 1.73 × 105 | 7,550 |
| Mineral extraction | MJ surplus | 2.34 × 103 | 2.7 |

3.4. Sensitivity Analysis Results
3.4.1. Vary Fraction of Nickel Oxide
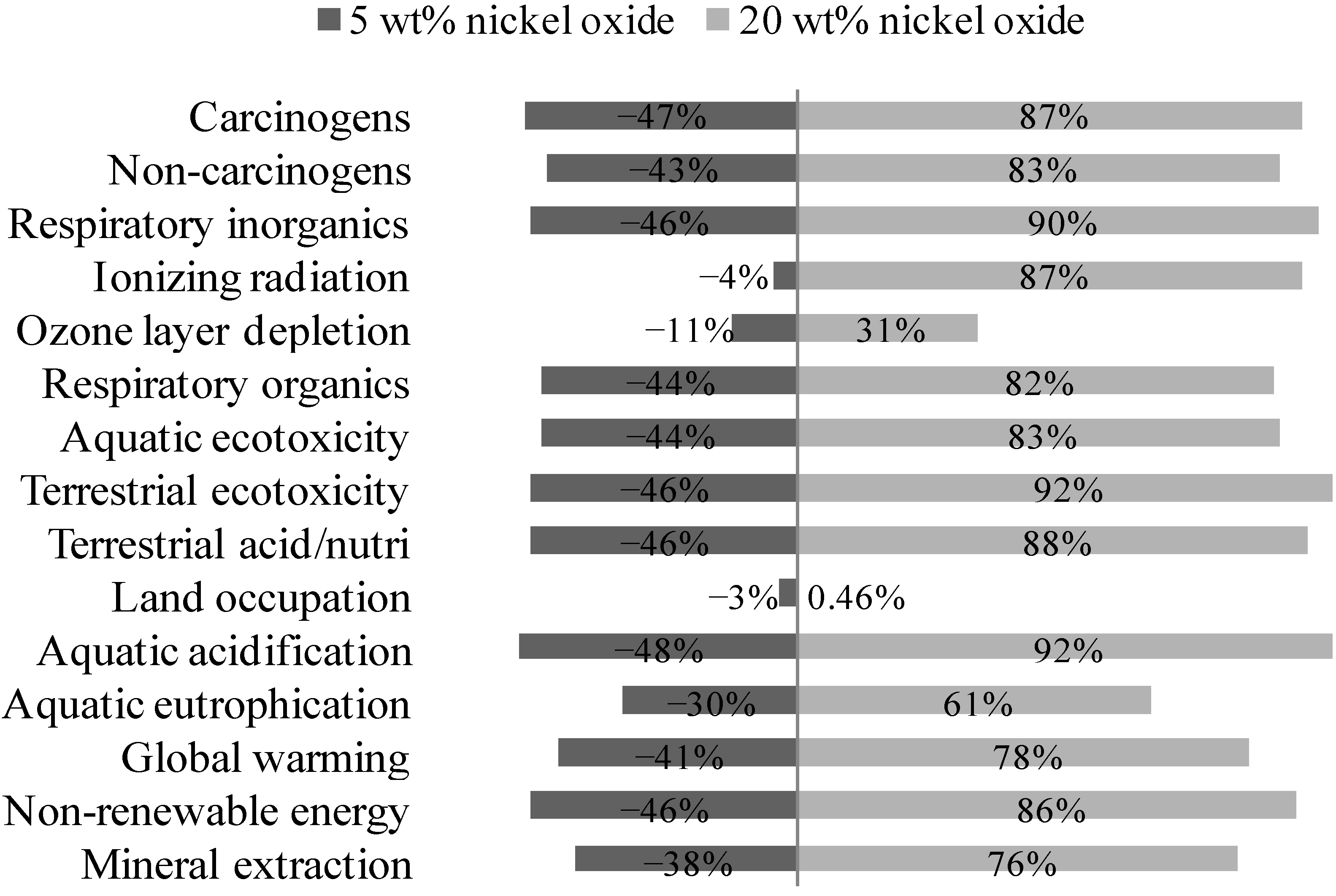
3.4.2. Vary Energy Used in Nickel Oxide Production
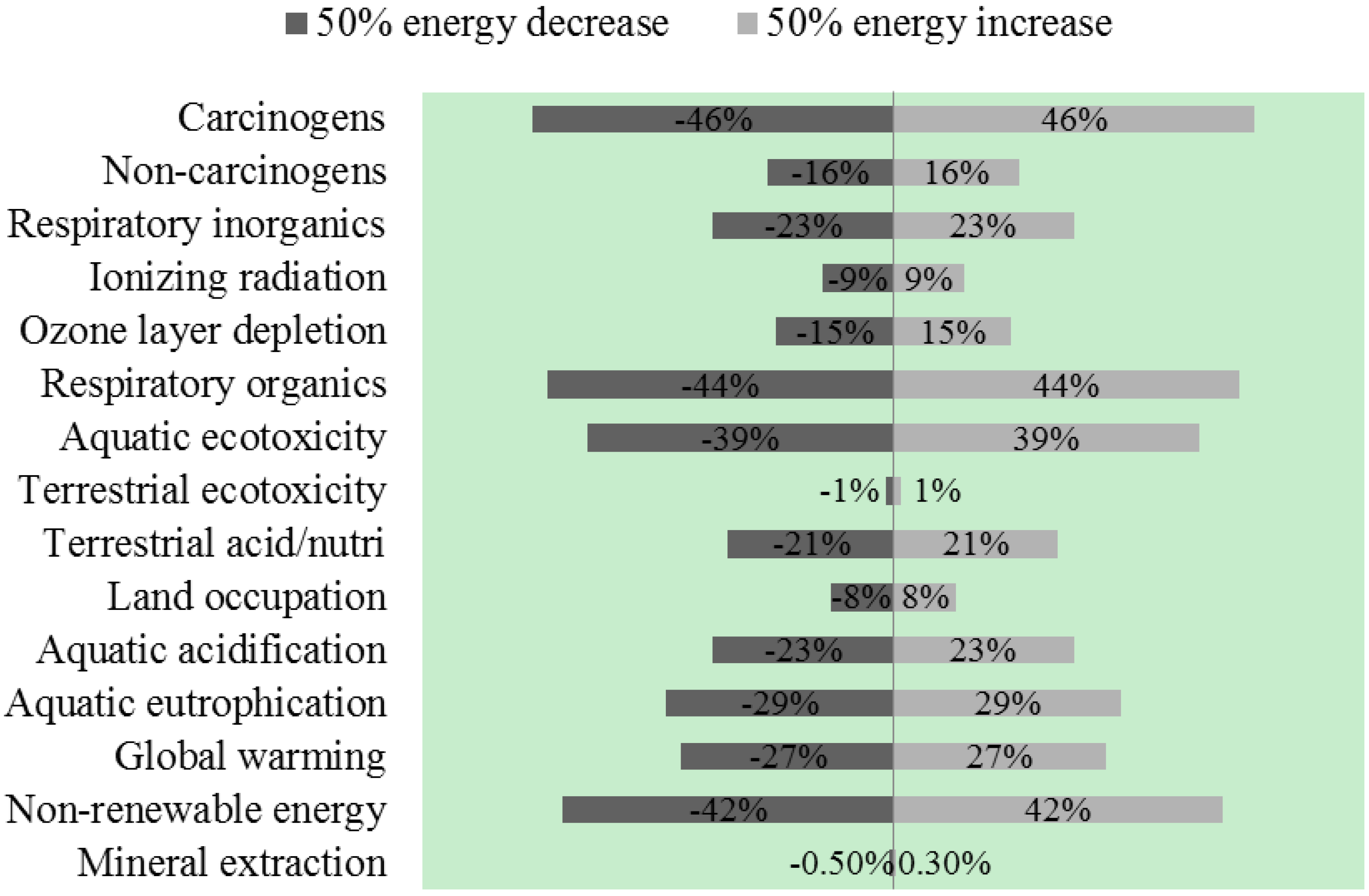
3.4.3. Vary Land Use for Switchgrass Production

3.4.4. Vary Switchgrass Yield
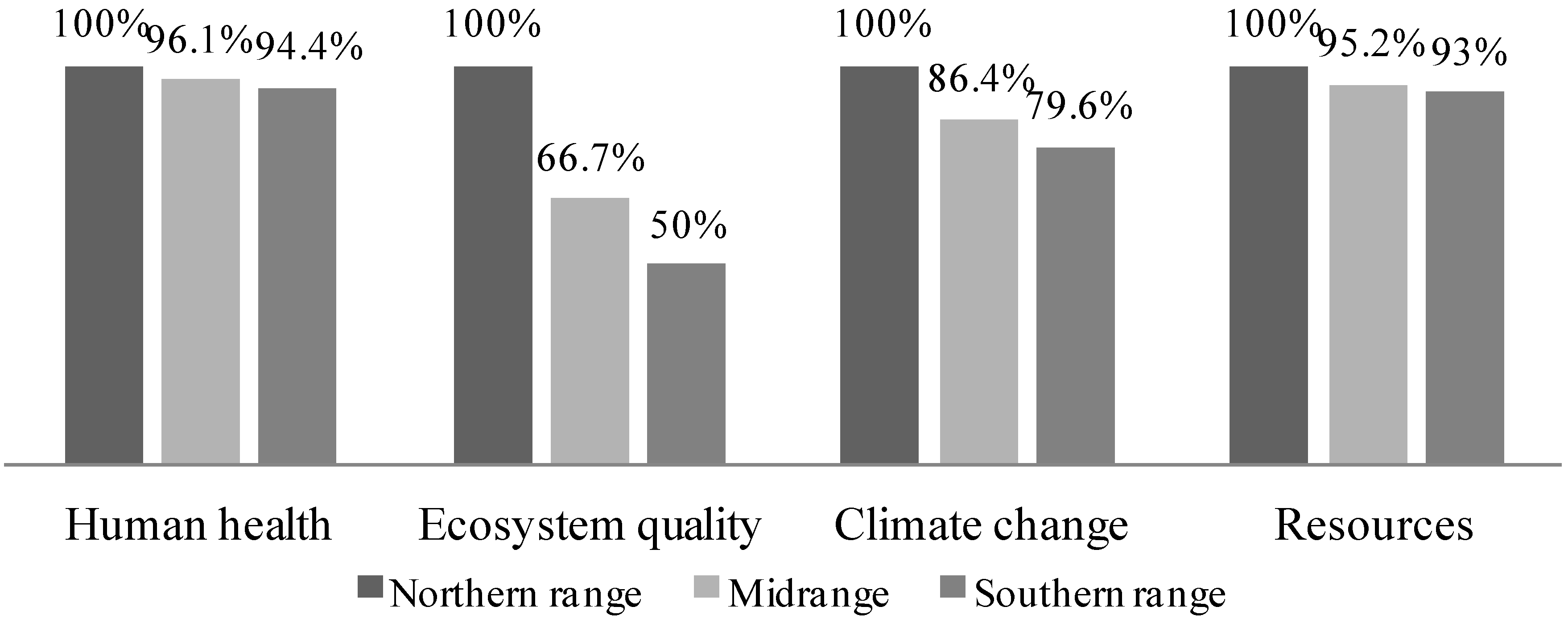
3.4.5. Vary Gasification Equivalence Ratio
| Inputs | ER | ||||
|---|---|---|---|---|---|
| 0.20 | 0.29 | 0.32 | 0.40 | 0.45 | |
| Air (kg) | 0.96 | 0.95 | 0.956 | 1 | 1.08 |
| Biomass energy (MJ) | 15.7 | 11 | 9.45 | 8.56 | 7.53 |
| Biomass mass (kg) | 0.83 | 0.59 | 0.5 | 0.45 | 0.4 |
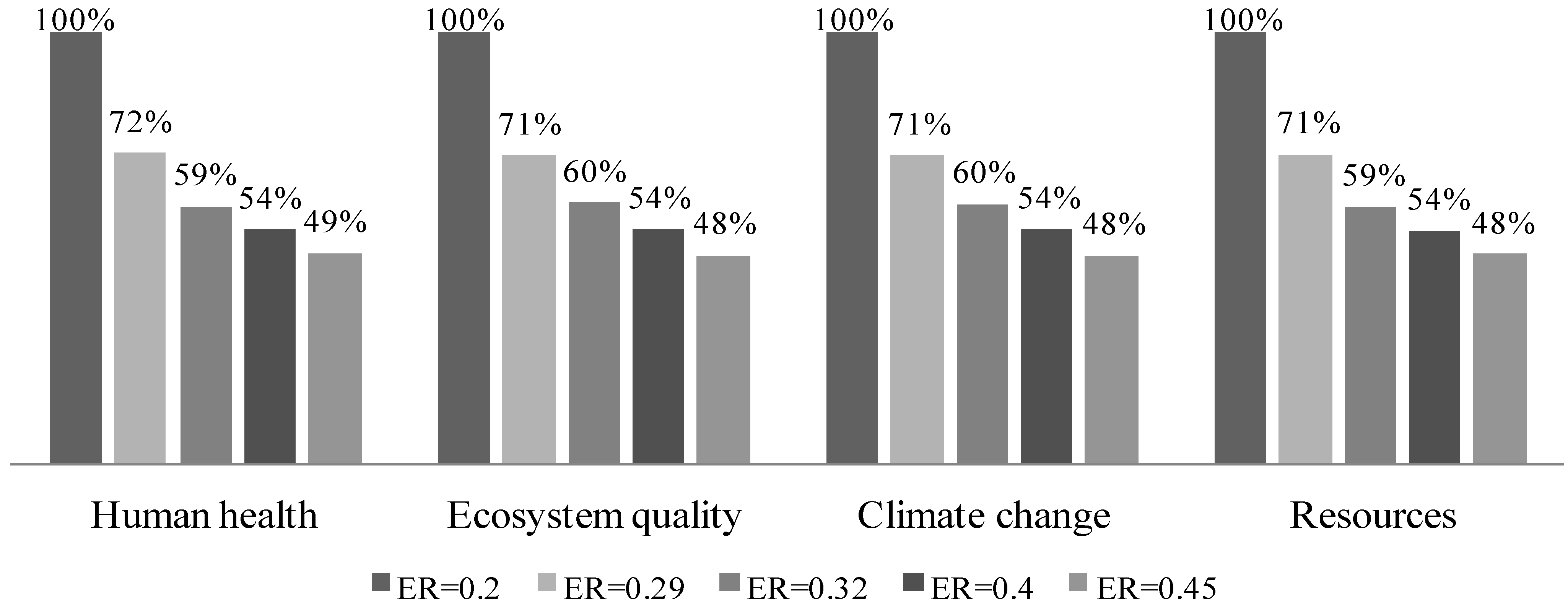
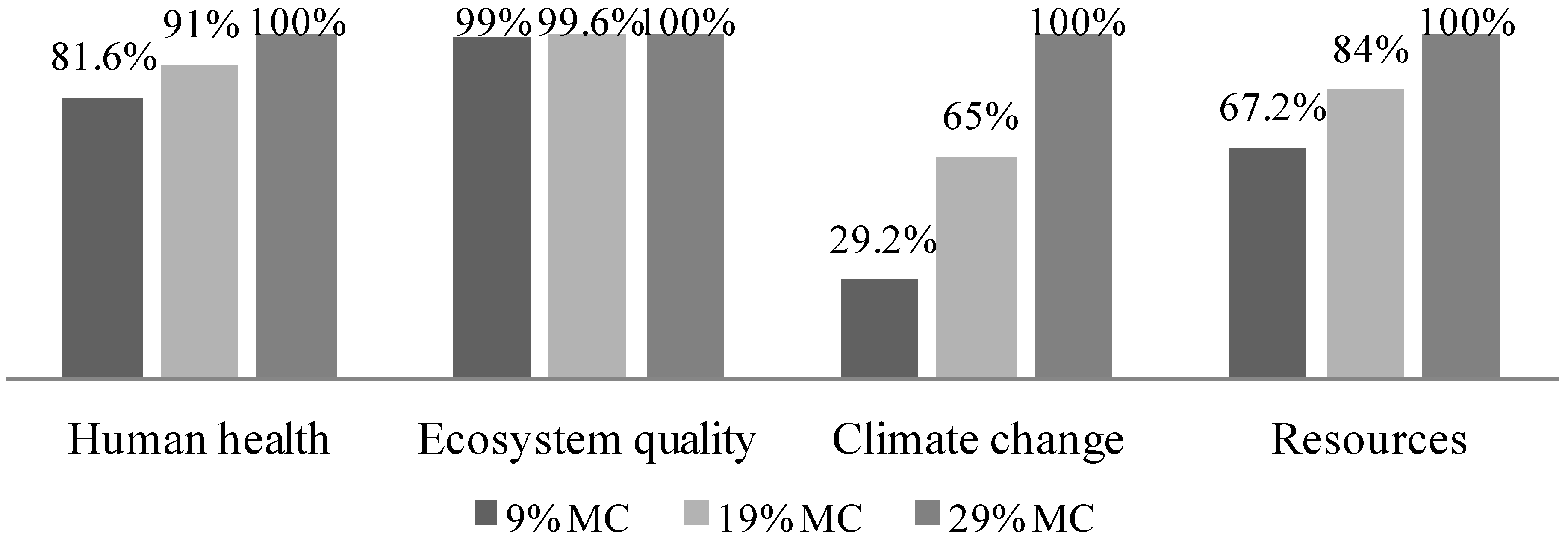
3.4.6. Vary Biomass Moisture Content Fed to the Gasifier
| MC (% wet basis) | Gasification products (% feed weight) | ||||||
|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | H2O | Tar | Ash | |
| 9 | 0.90 | 37.91 | 5.74 | 55.92 | 17.71 | 2.81 | 8.94 |
| 19 | 0.59 | 34.54 | 4.62 | 51.07 | 20.26 | 2.14 | 8.47 |
| 29 | 0.43 | 29.42 | 3.41 | 50.01 | 21.06 | 1.62 | 8.28 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caputo, A.C.; Palumbo, M.; Pelagagge, P.M.; Scacchia, F. Economics of biomass energy utilization in combustion and gasification plants: Effects of logistic variables. Biomass Bioenergy 2005, 28, 35–51. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Alauddin, Z.A.B.Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review. Renew. Sustain. Energy Rev. 2010, 14, 2852–2862. [Google Scholar] [CrossRef]
- Pereira, E.G.; da Silva, J.N.; de Oliveira, J.L.; Machado, C.S. Sustainable energy: A review of gasification technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Corti, A.; Lombardi, L. Biomass integrated gasification combined cycle with reduced CO2 emissions: Performance analysis and life cycle assessment (LCA). Energy 2004, 29, 2109–2124. [Google Scholar] [CrossRef]
- Koroneos, C.; Dompros, A.; Roumbas, G. Hydrogen production via biomass gasification—A life cycle assessment approach. Chem. Eng. Process. Process Intensif. 2008, 47, 1261–1268. [Google Scholar] [CrossRef]
- Gerber, M.A. Review of Novel Catalysts for Biomass Tar Cracking and Methane Reforming; Pacific Northwest National Laboratory: Richland, WA, USA, 2007. [Google Scholar]
- Mani, S.; Kastner, J. Catalytic cracking of tar using biochar as a catalyst. In Proceedings of the 2010 ASABE Annual International Meeting, Pittsburgh, PA, USA, 20–23 June 2010.
- Kimura, T.; Miyazawa, T.; Nishikawa, J.; Kado, S.; Okumura, K.; Miyao, T.; Naito, S.; Kunimori, K.; Tomishige, K. Development of Ni catalysts for tar removal by steam gasification of biomass. Appl. Catal. B Environ. 2006, 68, 160–170. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar] [CrossRef]
- Sohi, S.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1. [Google Scholar] [CrossRef]
- Schahczenski, J. Biochar and Sustainable Agriculture; ATTRA—National Sustainable Agriculture Information Service: Butte, MT, USA, 2010. [Google Scholar]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2009, 44, 827–833. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Syu, F.-S.; Chiueh, P.-T.; Lo, S.-L. Life cycle assessment of biochar cofiring with coal. Bioresour. Technol. 2013, 131, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sparrevik, M.; Field, J.L.; Martinsen, V.; Breedveld, G.D.; Cornelissen, G. Life cycle assessment to evaluate the environmental impact of biochar implementation in conservation agriculture in Zambia. Environ. Sci. Technol. 2013, 47, 1206–1215. [Google Scholar] [PubMed]
- Bojacá, C.R.; Schrevens, E. Parameter uncertainty in LCA: Stochastic sampling under correlation. Int. J. Life Cycle Assess. 2010, 15, 238–246. [Google Scholar] [CrossRef]
- Vigon, B.W.; Jensen, A.A. Life cycle assessment: Data quality and databases practitioner survey. J. Clean. Prod. 1995, 3, 135–141. [Google Scholar] [CrossRef]
- Huijbregts, M.A. Application of uncertainty and variability in LCA. Int. J. Life Cycle Assess. 1998, 3, 273–280. [Google Scholar] [CrossRef]
- Ardente, F.; Beccali, G.; Cellura, M.; Lo Brano, V. Life cycle assessment of a solar thermal collector: Sensitivity analysis, energy and environmental balances. Renew. Energy 2005, 30, 109–130. [Google Scholar] [CrossRef]
- Baker, J.W.; Lepech, M.D. Treatment of uncertainties in life cycle assessment. In Proceedings of the ICOSSAR 2009—10th International Conference on Structural Safety and Reliability, Osaka, Japan, 13–17 September 2009.
- Brown, T.R.; Zhang, Y.N.; Hu, G.P.; Brown, R.C. Techno-economic analysis of biobased chemicals production via integrated catalytic processing. Biofuels Bioprod. Biorefining 2012, 6, 73–87. [Google Scholar] [CrossRef]
- Marin, L.S. Treatment of Biomass-Derived Synthesis Gas Using Commercial Steam Reforming Catalysts and Biochar. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2011. [Google Scholar]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Progress Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Spath, P.L.; Mann, M.K.; Kerr, D.R. Life Cycle Assessment of Coal-Fired Power Production; National Renewable Energy Laboratory: Golden, CO, USA, 1999. [Google Scholar]
- National Renewable Energy Laboratory. U.S. Life Cycle Inventory Database. 2012. Available online: https://www.lcacommons.gov/nrel/search 2012 (accessed on 19 November 2012). [Google Scholar]
- Jolliet, O.; Margni, M.; Charles, R.; Humbert, S.; Payet, J.; Rebitzer, G.; Rosenbaum, R. IMPACT 2002+: A new life cycle impact assessment methodology. Int. J. Life Cycle Assess. 2003, 8, 324–330. [Google Scholar] [CrossRef]
- Bare, J.C.; Hofstetter, P.; Pennington, D.W.; de Haes, H.A.U. Midpoints versus endpoints: The sacrifices and benefits. Int. J. Life Cycle Assess. 2000, 5, 319–326. [Google Scholar] [CrossRef]
- Sharma, A.M.; Kumar, A.; Patil, K.N.; Huhnke, R.L. Performance evaluation of a lab-scale fluidized bed gasifier using switchgrass as feedstock. Trans. ASABE 2011, 54, 2259–2266. [Google Scholar] [CrossRef]
- Life Cycle Assessment of Nickel Products; Ecobalance, Inc.: Bethesda, MD, USA, 2000; pp. 1–99.
- US-EI SimaPro Database; EarthShift, LLC: Huntington, VT, USA, 2013.
- Kiss, E.; Bošković, G.; Lazić, M.; Lomić, G.; Marinković-Nedučin, R. The morphology of the NiO-Al2O3 catalyst. Scanning 2006, 28, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Gorokhov, V.; Manfredo, L.; Ratafia-Brown, J.; Ramezan, M.; Stiegel, G. Life cycle assessment of gasification-based power cycles. In Proceedings of the 2000 International Joint Power Generation Conference, Miami Beach, FL, USA, 23–26 July 2000.
- Goedkoop, M.; de Schryver, A.; Oele, M.; Durksz, S.; de Roest, D. Introduction to LCA with SimaPro 7; PRé Consultants: Amersfoort, The Netherlands, 2008. [Google Scholar]
- Norgate, T.; Jahanshahi, S. Assessing the energy and greenhouse gas footprints of nickel laterite processing. Miner. Eng. 2011, 24, 698–707. [Google Scholar] [CrossRef]
- Eckelman, M.J. Facility-level energy and greenhouse gas life-cycle assessment of the global nickel industry. Resour. Conserv. Recycl. 2010, 54, 256–266. [Google Scholar] [CrossRef]
- Coogan, T.P.; Latta, D.M.; Snow, E.T.; Costa, M.; Lawrence, A. Toxicity and carcinogenicity of nickel compounds. CRC Crit. Rev. Toxicol. 1989, 19, 341–384. [Google Scholar] [CrossRef]
- Weisbrod, A.V.; van Hoof, G. LCA-measured environmental improvements in Pampers® diapers. Int. J. Life Cycle Assess. 2012, 17, 145–153. [Google Scholar] [CrossRef]
- Frischknecht, R.; Braunschweig, A.; Hofstetter, P.; Suter, P. Human health damages due to ionising radiation in life cycle impact assessment. Environ. Impact Assess. Rev. 2000, 20, 159–189. [Google Scholar] [CrossRef]
- Bai, Y.; Luo, L.; van der Voet, E. Life cycle assessment of switchgrass-derived ethanol as transport fuel. Int. J. Life Cycle Assess. 2010, 15, 468–477. [Google Scholar] [CrossRef]
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land clearing and the biofuel carbon debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.H. Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Van der Voet, E. Land Use in LCA; CML-SSP Working Paper 02.002; Section Substances and Products, Centre of Environmental Science, Leiden University (CML-SSP): Leiden, The Netherlands, 2001. [Google Scholar]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Dale, B.E. Cumulative energy and global warming impact from the production of biomass for biobased products. J. Ind. Ecol. 2003, 7, 147–162. [Google Scholar] [CrossRef]
- Liebig, M.A.; Schmer, M.R.; Vogel, K.P.; Mitchell, R.B. Soil carbon storage by switchgrass grown for bioenergy. Bioenergy Res. 2008, 1, 215–222. [Google Scholar] [CrossRef]
- Humbert, S.; de Schryver, A.; Margni, M.; Jolliet, O. IMPACT 2002+: User Guide; Draft for Version Q2.21; Industrial Ecology & Life Cycle Systems Group, GECOS, Swiss Federal Institute of Technology Lausanne (EPFL): Lausanne, Switzerland, 2005. [Google Scholar]
- Grierson, S.; Strezov, V.; Bengtsson, J. Life cycle assessment of a microalgae biomass cultivation, bio-oil extraction and pyrolysis processing regime. Algal Res. 2013, 2, 299–311. [Google Scholar] [CrossRef]
- Worley, M.; Yale, J. Biomass Gasification Technology Assessment: Consolidated Report; NREL/SR-5100-57085; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazier, R.S.; Jin, E.; Kumar, A. Life Cycle Assessment of Biochar versus Metal Catalysts Used in Syngas Cleaning. Energies 2015, 8, 621-644. https://doi.org/10.3390/en8010621
Frazier RS, Jin E, Kumar A. Life Cycle Assessment of Biochar versus Metal Catalysts Used in Syngas Cleaning. Energies. 2015; 8(1):621-644. https://doi.org/10.3390/en8010621
Chicago/Turabian StyleFrazier, Robert S., Enze Jin, and Ajay Kumar. 2015. "Life Cycle Assessment of Biochar versus Metal Catalysts Used in Syngas Cleaning" Energies 8, no. 1: 621-644. https://doi.org/10.3390/en8010621





