Didemnaketals F and G, New Bioactive Spiroketals from a Red Sea Ascidian Didemnum Species
Abstract
:1. Introduction
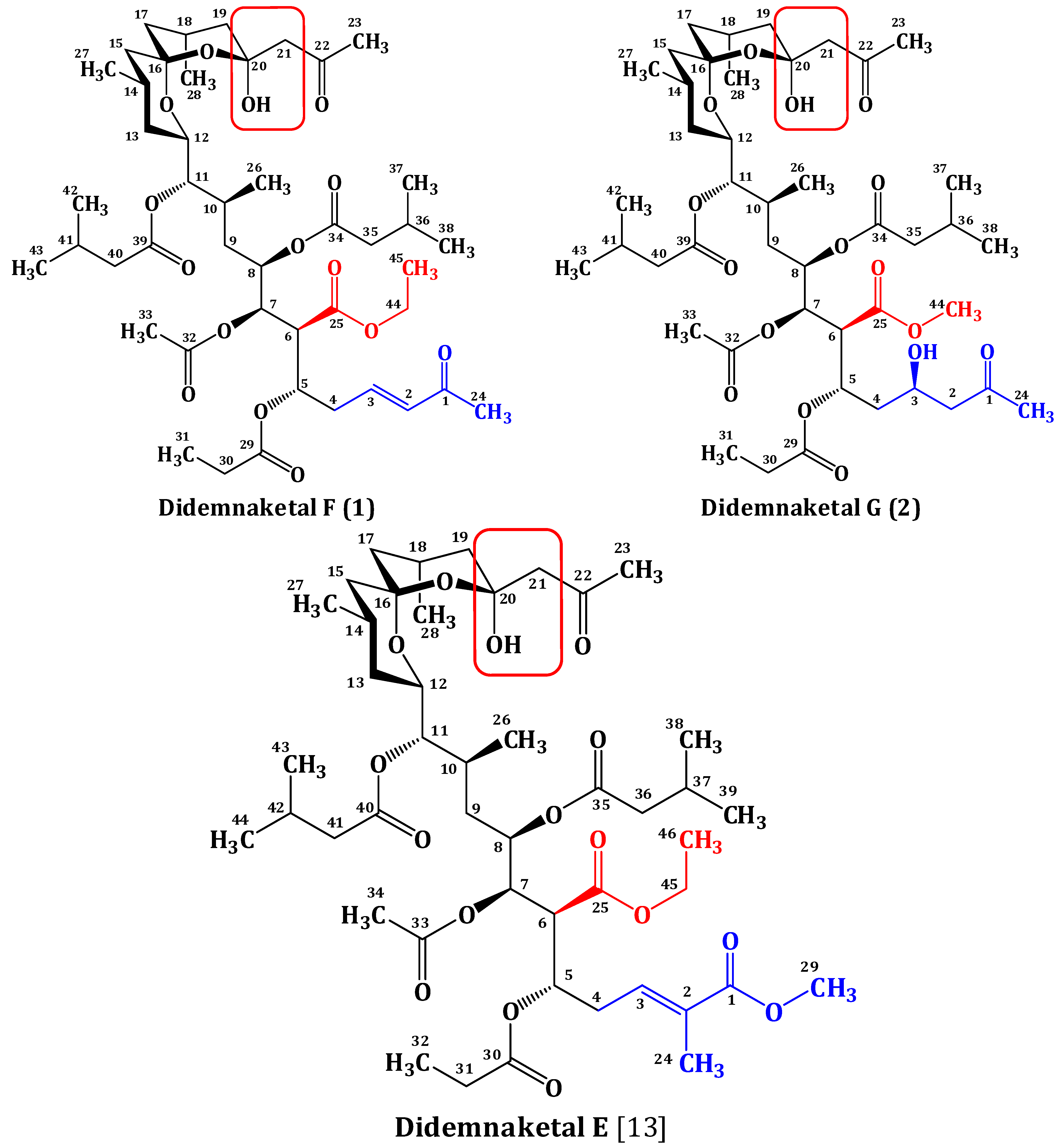
2. Results and Discussion
2.1. Purification of Compounds 1 and 2
2.2. Structure Elucidation of Compound 1
| No. | δC, m a | δH m (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 198.5, qC | - | - | - |
| 2 | 133.0, CH | 6.11 d (16.5) | 3 | - |
| 3 | 145.8, CH | 6.75 dt (16.5, 6.8) | 2, 4 | 1 |
| 4 | 29.9, CH2 | 1.97 m | 3, 5 | 2, 6, 25 |
| 5 | 72.3, CH | 3.45dt (11.6, 7.3) | 4, 6 | 6, 7, 25, 29 |
| 6 | 41.6, CH | 2.30 dt (11.6, 6.9) | 5, 7 | 4, 25, 32 |
| 7 | 67.9, CH | 4.83 t (9.6) | 6, 8 | 5, 9, 32 |
| 8 | 67.5, CH | 4.30 m | 7, 9 | 6, 7, 10, 34 |
| 9 | 36.8, CH2 | 1.70 m, 1.25 m | 8, 10 | 7, 8, 10 |
| 10 | 34.1, CH | 1.92 m | 9, 26 | 8, 12 |
| 11 | 76.5, CH | 5.02 dd (6.8, 6.3) | 10, 12 | 9, 12, 39 |
| 12 | 75.3, CH | 3.86 m | 11, 13 | 10, 11, 16 |
| 13 | 36.1, CH2 | 1.48 m, 0.88 m | 12, 14 | 11, 14, 15 |
| 14 | 25.5, CH | 1.72 m | 13, 15 | 12, 16 |
| 15 | 44.8, CH2 | 1.62 m, 1.08 m | 14 | 16, 17 |
| 16 | 98.7, qC | - | - | - |
| 17 | 42.0, CH2 | 1.72 m, 1.56 m | 18 | 15, 16 |
| 18 | 24.6, CH | 1.75 m | 17, 19, 28 | 16, 20 |
| 19 | 33.1, CH2 | 2.15 m, 1.74 m | 18 | 17, 20 |
| 20 | 99.8, qC | - | - | - |
| 21 | 41.2, CH2 | 2.95 m, 2.86 m | - | 20, 22 |
| 22 | 202.5, qC | - | - | - |
| 23 | 21.3, CH3 | 2.19 s | - | 22 |
| 24 | 29.7, CH3 | 2.24 s | - | 1 |
| 25 | 172.6, qC | - | - | - |
| 26 | 14.8, CH3 | 0.90 d (6.5) | 10 | 9, 10 |
| 27 | 22.2, CH3 | 0.91 d (6.8) | 14 | 13, 14, 15, 16 |
| 28 | 19.8, CH3 | 0.97 d (6.5) | 18 | 18, 19, 20 |
| 29 | 170.5, qC | - | - | - |
| 30 | 26.6, CH2 | 2.16 q (7.3) | 31 | 29, 31 |
| 31 | 7.5, CH3 | 1.03 t (7.3) | 30 | 29, 30 |
| 32 | 170.2, qC | - | - | - |
| 33 | 21.0, CH3 | 2.05 s | - | 32 |
| 34 | 176.6, qC | - | - | - |
| 35 | 41.8, CH2 | 2.13 m | 36 | 37, 38 |
| 36 | 33.9, CH | 2.87 m | 35, 37, 38 | 34, 35 |
| 37 | 17.2, CH3 | 1.20 d (6.8) | 36 | 34, 35, 36 |
| 38 | 17.2, CH3 | 1.20 d (6.8) | 36 | 34, 35, 36 |
| 39 | 168.6, qC | - | - | - |
| 40 | 33.2, CH2 | 2.03 m | 41 | 39, 41 |
| 41 | 31.2, CH | 1.97 m | 40, 42 | 39, 42, 43 |
| 42 | 22.0, CH3 | 0.95 d (6.3) | 41 | 40, 41, 43 |
| 43 | 22.0, CH3 | 0.95 d (6.3) | 41 | 40, 41, 42 |
| 44 | 60.4, CH2 | 4.11 q (6.8) | 45 | 25, 45 |
| 45 | 14.3, CH3 | 1.27 t (6.8) | 44 | 44 |

| No. | δC, m a | δH m (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 207.2, qC | - | - | - |
| 2 | 50.1, CH2 | 2.61 dd (14.5, 7.3) 2.45 dd (14.5, 7.5) | 3 | 1, 3, 4 |
| 3 | 67.5, CH | 4.28 quin (7.3) | 2, 4 | 1, 2 |
| 4 | 36.1, CH2 | 1.65 m, 0.87 m | 3, 5 | 2, 3, 5 |
| 5 | 72.3, CH | 3.45dt (11.5, 6.9) | 4, 6 | 3, 6, 7, 25, 29 |
| 6 | 41.7, CH | 2.95 dt (11.6, 6.5) | 5, 7 | 4, 8, 25 |
| 7 | 67.9, CH | 4.83 t (9.8) | 6, 8 | 5, 9, 32 |
| 8 | 67.5, CH | 3.90 m | 7, 9 | 6, 7, 9, 34 |
| 9 | 36.9, CH2 | 1.75 m, 1.29 m | 8, 10 | 7, 8, 10, 26 |
| 10 | 33.9, CH | 1.95 m | 9, 11, 26 | 8, 11, 26, 39 |
| 11 | 76.8, CH | 5.01 dd (6.5, 6.3) | 10, 12 | 9, 10, 12, 39 |
| 12 | 75.3, CH | 3.86 m | 11, 13 | 10, 14, 16 |
| 13 | 36.1, CH2 | 1.47 m, 0.85 m | 12, 14 | 11, 15 |
| 14 | 26.6, CH | 1.70 m | 13, 15 | 12, 16 |
| 15 | 44.7,CH2 | 1.63 m, 1.08 m | 14 | 13, 16 |
| 16 | 98.7, qC | - | - | - |
| 17 | 41.1, CH2 | 1.74 m, 1.55 m | 18 | 15, 16, 19 |
| 18 | 24.6, CH | 1.75 m | 17, 19, 28 | 16, 20, 28 |
| 19 | 32.0, CH2 | 2.15 m | 18 | 20, 21 |
| 20 | 99.8, qC | - | - | - |
| 21 | 41.1, CH2 | 2.85 m, 2.72 m | - | 20, 22, 23 |
| 22 | 202.5, C | - | - | - |
| 23 | 21.0, CH3 | 2.06 s | - | 22 |
| 24 | 31.2, CH3 | 2.16 s | - | 1, 2 |
| 25 | 175.8, qC | - | - | - |
| 26 | 14.8, CH3 | 0.88 d (6.5) | 10 | 9, 10, 11 |
| 27 | 22.0, CH3 | 0.92 d (6.8) | 14 | 12, 13, 14 |
| 28 | 20.5, CH3 | 0.90 d (6.5) | 18 | 17, 18, 19, 20 |
| 29 | 170.2, qC | - | - | - |
| 30 | 26.8, CH2 | 2.18 q (7.3) | 31 | 29, 31 |
| 31 | 7.5, CH3 | 1.03 t (6.9) | 30 | 29, 30 |
| 32 | 170.5, qC | - | - | - |
| 33 | 21.3, CH3 | 2.01 s | - | 32 |
| 34 | 176.6, qC | - | - | - |
| 35 | 39.8, CH2 | 2.33 m | 36 | 34, 37, 38 |
| 36 | 33.2, CH | 2.85 m | 35, 37, 38 | 34 |
| 37 | 17.2, CH3 | 1.22 d (6.8) | 36 | 34, 35, 36 |
| 38 | 17.2, CH3 | 1.22 d (6.8) | 36 | 34, 35, 36 |
| 39 | 168.6, qC | - | - | - |
| 40 | 33.1, CH2 | 2.05 m | 41 | 39, 41 |
| 41 | 29.7, CH | 1.97 m | 40, 42 | 39, 42, 43 |
| 42 | 22.2, CH3 | 0.93 d (6.3) | 41 | 40, 41, 43 |
| 43 | 22.2, CH3 | 0.93 d (6.3) | 41 | 40, 41, 42 |
| 44 | 51.8, CH3 | 3.64 s | - | 25 |
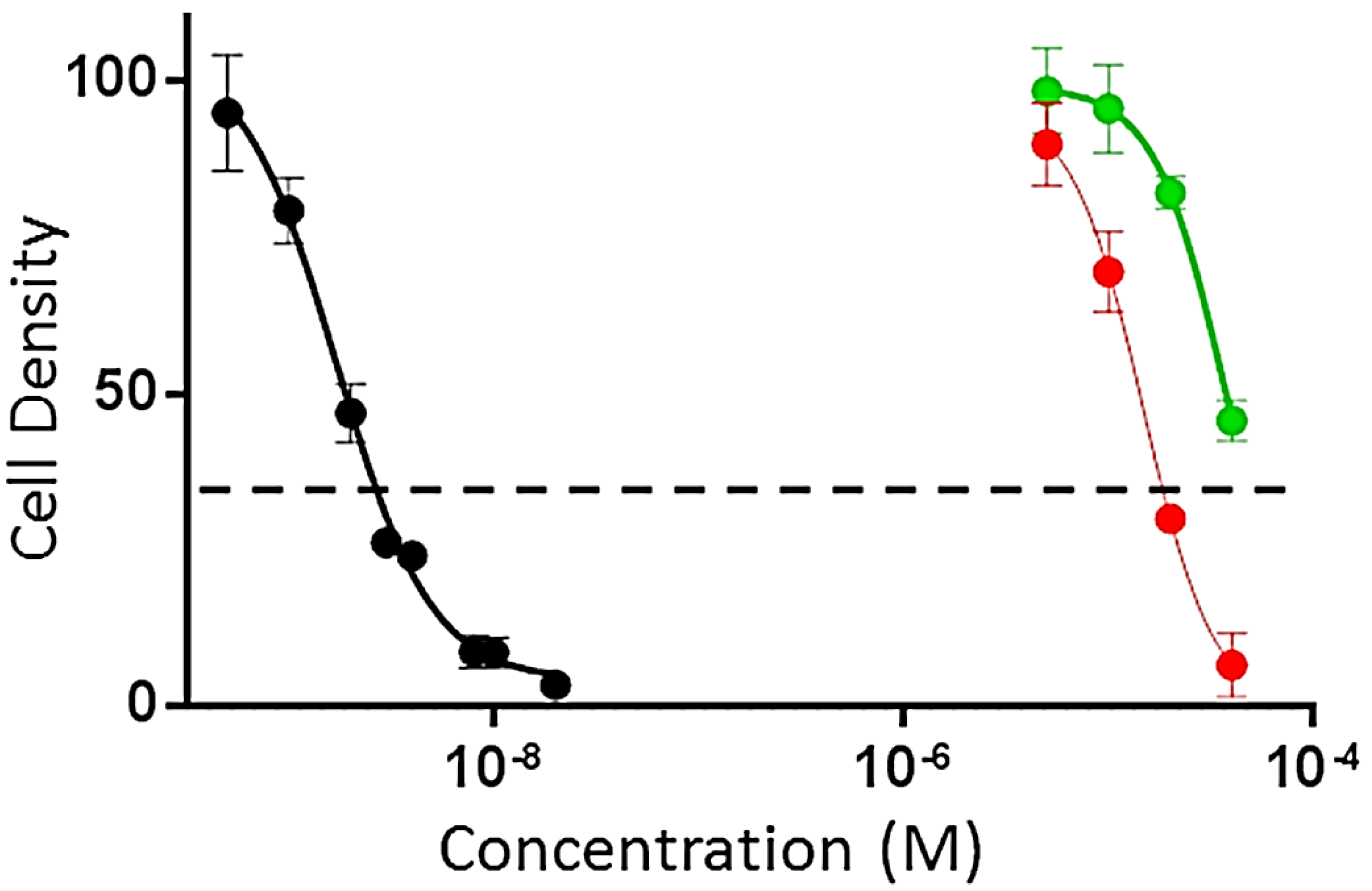
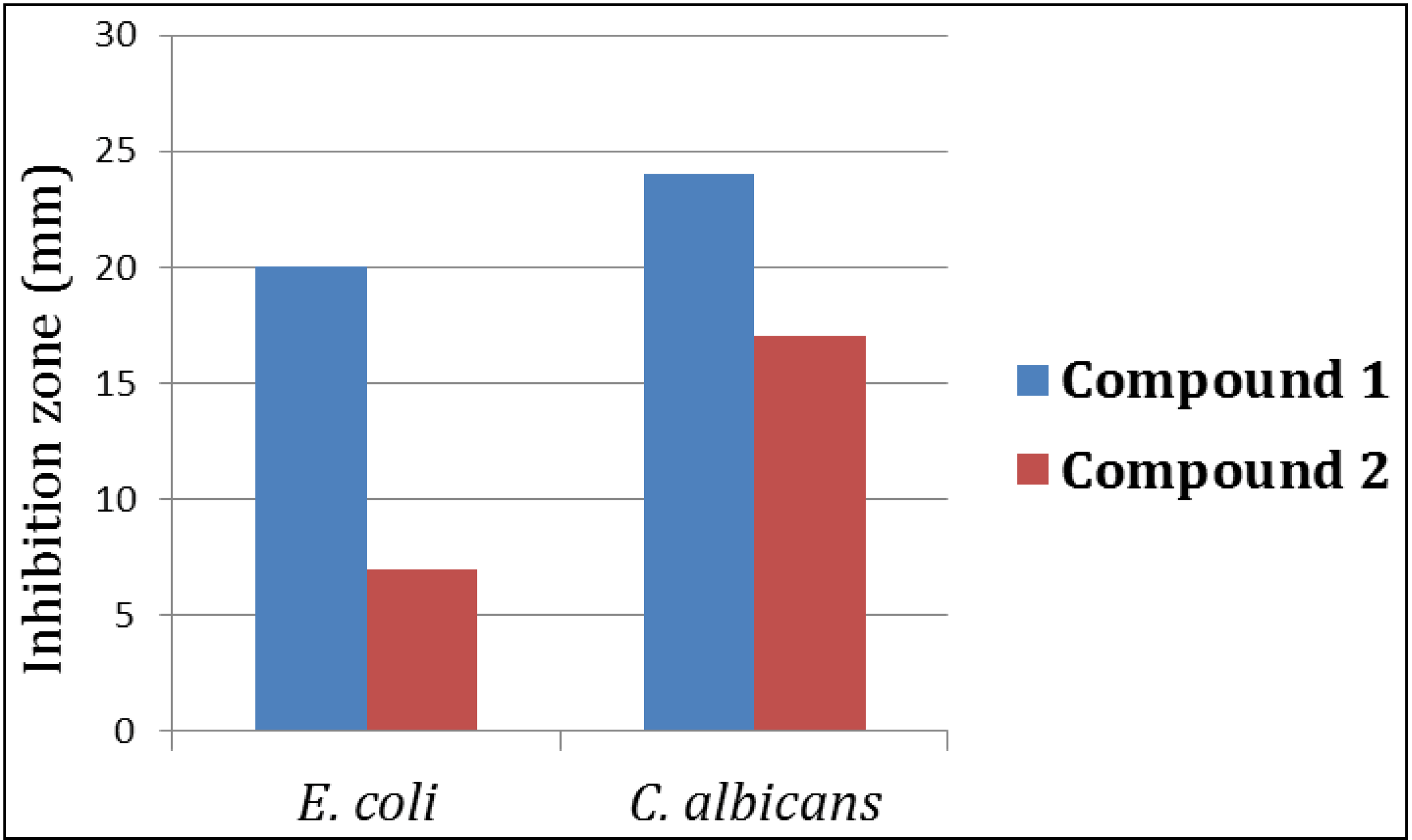
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Extraction and Purifications of Compounds 1 and 2
3.4. Biological Evaluation of the Compounds
3.4.1. Evaluation of Antiproliferaive and Cytotoxic Activities
3.4.2. Evaluation of the Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koehn, F.E.; Carter, G.T. The evolving role of natural products drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products from marine invertebrates and microbes as modulators of antitumor targets. Curr. Drug Targets 2006, 7, 279–304. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Bedford, J.J. The incidence of antibacterial activity in marine demospongiae: Systematic and geographic considerations. Mar. Biol. 1978, 46, 215–221. [Google Scholar] [CrossRef]
- Roy, R.S.; Gehring, A.M.; Milne, J.C.; Belshaw, P.J.; Walsh, C.T. Thiazole and oxazole peptides: Biosynthesis and molecular machinery. Nat. Prod. Rep. 1999, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Toshiaki, T.; Hiroaki, S.; Kiyotake, S. Hexamollamide, a hexapeptide from an Okinawan ascidian Didemnum molle. Tetrahedron Lett. 2008, 49, 5297–5299. [Google Scholar] [CrossRef]
- Molinski, T.F. Marine pyridoacridine alkaloids: Structure, synthesis, and biological chemistry. Chem. Rev. 1993, 93, 1825–1838. [Google Scholar] [CrossRef]
- Schumacher, R.W.; Davidson, B.S. Didemnolines A–D, new N9-substituted β-carbolines from the marine ascidian Didemnum sp. Tetrahedron 1995, 51, 10125–10130. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Reddy, V.L.N.; Venkataramana, G.; Ravinder, K.; Srinivasulu, M.; Raju, T.V.; Ravikumar, K.; Chandrasekar, D.; Ramakrishna, S.; Venkateswarlu, Y. New lamellarin alkaloids from the Indian ascidian Didemnum obscurum and their antioxidant properties. J. Nat. Prod. 2004, 67, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, H.C.; Pawlik, J.R.; Fenical, W. Chemical defense of the Caribbean ascidian Didemnum conchyliatum. Mar. Ecol. Prog. Ser. 1998, 164, 221–228. [Google Scholar] [CrossRef]
- Davis, R.A.; Sandoval, I.T.; Concepcion, G.P.; da Rocha, R.M.; Ireland, C.M. Lissoclinotoxins E and F, novel cytotoxic alkaloids from a Philippine didemnid ascidian. Tetrahedron 2003, 59, 2855–2859. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Badr, J.M.; Youssef, D.T.A. Didemnaketals D and E, bioactive terpenoids from a Red Sea ascidian Didemnum species. Tetrahedron 2014, 70, 35–40. [Google Scholar] [CrossRef]
- Pika, J.; Faulkner, D.J. A reinvestigation of the didemnaketals from the Palauan ascidian Didemnum sp. Nat. Prod. Lett. 1995, 7, 291–296. [Google Scholar] [CrossRef]
- Potts, B.C.M.; Faulkner, D.J. Didemnaketals A and B, HIV-1 protease inhibitors from the ascidian Didemnum sp. J. Am. Chem. Soc. 1991, 113, 6321–6322. [Google Scholar] [CrossRef]
- Choi, S.; Cha, B.; Kagami, I.; Lee, Y.; Sasaki, H.; Suenaga, K.; Teruya, T.; Yonezawa, T.; Nagai, K.; Woo, J. N,N′-diphenethylurea isolated from Okinawan ascidian Didemnum molle enhances adipocyte differentiation in 3T3-L1 cells. J. Antibiotic 2011, 64, 277–280. [Google Scholar] [CrossRef]
- Wright, A.D.; Goclik, E.; König, G.M.; Kaminsky, R. Antiplasmodial and antitrypanosomal decahydroquinoline derivatives from the tropical marine tunicate Didemnum sp. J. Med. Chem. 2002, 45, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Flentke, G.R.; Rich, D.H. Inhibition of HIV-1 protease by a subunit of didemnaketal A. J. Am. Chem. Soc. 1998, 120, 8893–8894. [Google Scholar] [CrossRef]
- Mitchell, S.S.; Rhodes, D.; Bushman, F.D.; Faulkner, D.J. Cyclodidemniserinol trisulfate, a sulfated serinolipid from the Palauan ascidian Didemnum guttatum that inhibits HIV-1 integrase. Org. Lett. 2000, 2, 1605–1607. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Lopez, S.; Johnson, T.A.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Crews, P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla-Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Lett. 2003, 44, 3471–3475. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Glasser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar]
- A Service of the U.S. National Institutes of Health. Available online: https://clinicaltrials.gov/ (accessed on 20 July 2014).
- Shaala, L.A.; Bamane, F.H.; Badr, J.M.; Youssef, D.T.A. Brominated arginine-derived alkaloids from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2011, 74, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Noji, S.; Sasaki, M. Convergent assembly of the spiroacetal subunit of didemnaketal B. Org. Lett. 2010, 12, 5354–5357. [Google Scholar]
- Ito, H.; Inoue, T.; Iguchi, K. Toward the synthesis of didemnaketal B: A convergent synthesis of the C9-C28 subunit. Org. Lett. 2008, 10, 3873–3876. [Google Scholar] [CrossRef]
- Salomon, C.E.; Williams, D.H.; Lobkovsky, E.; Clardy, J.C.; Faulkner, D.J. Relative and absolute stereochemistry of the didemnaketals, metabolites of a Palauan ascidian Didemnum sp. Org. Lett. 2002, 4, 1699–1702. [Google Scholar]
- Fuwa, H.; Muto, T.; Sekine, K.; Sasaki, M. Total synthesis and structure revision of didemnaketal B. Chem. Eur. J. 2014, 20, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; LeBoeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Ludueña, R.F.; Kruh, G.D.; Mooberry, S.L. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G. Ecology and natural history of the protochordates. Can. J. Zool. 2005, 83, 34–50. [Google Scholar] [CrossRef]
- Lambert, G. Non indigenous ascidians in tropical waters. Pac. Sci. 2002, 56, 291–298. [Google Scholar] [CrossRef]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348, and references therein. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shaala, L.A.; Youssef, D.T.A.; Ibrahim, S.R.M.; Mohamed, G.A.; Badr, J.M.; Risinger, A.L.; Mooberry, S.L. Didemnaketals F and G, New Bioactive Spiroketals from a Red Sea Ascidian Didemnum Species. Mar. Drugs 2014, 12, 5021-5034. https://doi.org/10.3390/md12095021
Shaala LA, Youssef DTA, Ibrahim SRM, Mohamed GA, Badr JM, Risinger AL, Mooberry SL. Didemnaketals F and G, New Bioactive Spiroketals from a Red Sea Ascidian Didemnum Species. Marine Drugs. 2014; 12(9):5021-5034. https://doi.org/10.3390/md12095021
Chicago/Turabian StyleShaala, Lamiaa A., Diaa T.A. Youssef, Sabrin R.M. Ibrahim, Gamal A. Mohamed, Jihan M. Badr, April L. Risinger, and Susan L. Mooberry. 2014. "Didemnaketals F and G, New Bioactive Spiroketals from a Red Sea Ascidian Didemnum Species" Marine Drugs 12, no. 9: 5021-5034. https://doi.org/10.3390/md12095021







