Ontogenic Expression Pattern and Genetic Polymorphisms of the Fatty Acid Transport Protein 4 (FATP4) Gene in Chinese Chicken Populations
Abstract
:1. Introduction
2. Results
2.1. Expression of FATP4 among Chicken Tissues
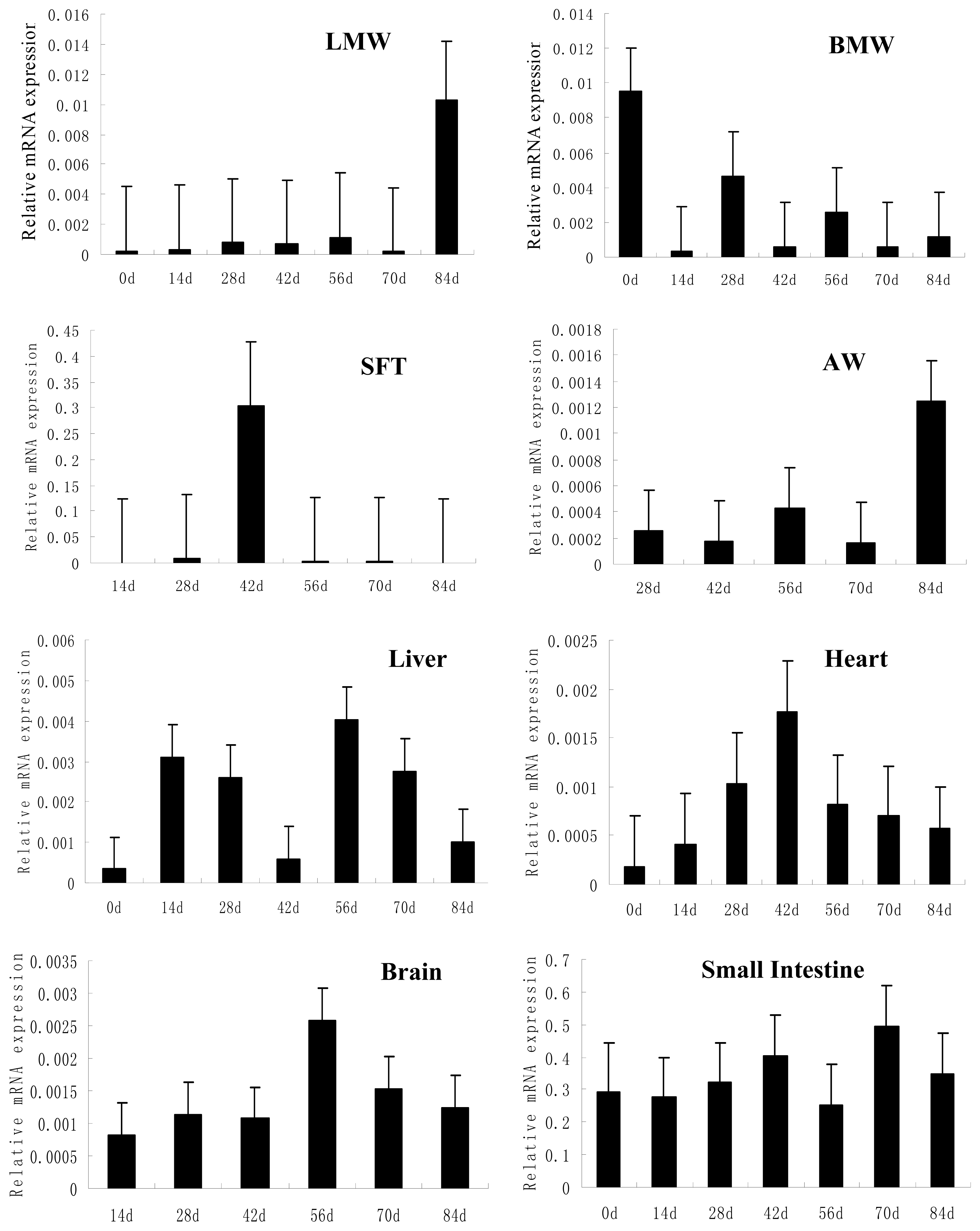
2.2. Ontogenic Expression of FATP4 in Chicken
2.3. Identification of Genetic Variants in Chicken FATP4 Gene
2.4. Association Analysis
3. Discussion
4. Experimental Section
4.1. Animal
4.2. Measurement of Carcass Traits
4.3. RNA Extraction, cDNA Synthesis and Quantification of FATP4 mRNA Expression
4.4. Identification of FATP4 Genetic Variants
4.5. Data Analysis
5. Conclusion
Acknowledgments
References
- Pohl, J.; Ring, A.; Hermann, T.; Stremmel, W. Role of FATP in parenchymal cell fatty acid uptake. Biochim. Biophys. Acta 2004, 1686, 1–6. [Google Scholar]
- Coburn, C.T.; Hajri, T.; Ibrahimi, A.; Abumrad, N.A. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J. Mol. Neurosci 2001, 16, 117–121. [Google Scholar]
- Glatz, J.F.C.; Luiken, J.J.F.P.; van Bilsen, M.; van der Vusse, G.J. Cellular lipid binding proteins as facilitators and regulators of lipid metabolism. Mol. Cell Biochem 2002, 239, 3–7. [Google Scholar]
- Huang, H.; Starodub, O.; McIntosh, A.; Kier, A.B.; Schroeder, F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J. Biol. Chem 2002, 277, 29139–29151. [Google Scholar]
- Stahl, A. A current review of fatty acid transport proteins (SLC27). Pflügers Arch 2004, 447, 722–727. [Google Scholar]
- Binnert, C.; Koistinen, H.A.; Martin, G.; Andreelli, F.; Ebeling, P.; Koivisto, V.A.; Laville, M.; Auwerx, J.; Vidal, H. Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am. J. Physiol. Endocrinol. Metab 2000, 279, E1072–E1079. [Google Scholar]
- Fisher, R.M.; Gertow, K. Fatty acid transport proteins and insulin resistance. Curr. Opin. Lipidol 2005, 16, 173–178. [Google Scholar]
- Pohl, J.; Fitscher, B.A.; Ring, A.; Ihl-Vahl, R.; Strasser, R.H.; Stremmel, W. Fatty acid transporters in plasma membranes of cardiomyocytes in patients with dilated cardiomyopathy. Eur. J. Med. Res 2000, 5, 438–442. [Google Scholar]
- Pei, Z.; Fraisl, P.; Berger, J.; Jia, Z.; Forss-Petter, S.; Watkins, P.A. Mouse very long-chain Acyl-CoA synthetase 3/fatty acid transport protein 3 catalyzes fatty acid activation but not fatty acid transport in MA-10 cells. J. Biol. Chem 2004, 279, 54454–54462. [Google Scholar]
- Stahl, A.; Gimeno, R.E.; Tartaglia, L.A.; Lodish, H.F. Fatty acid transport proteins: A current view of a growing family. Trends Endocrinol. Metab 2001, 12, 266–273. [Google Scholar]
- Gimeno, R.E. Fatty acid transport proteins. Curr. Opin. Lipidol 2007, 18, 271–276. [Google Scholar]
- Gimeno, R.E.; Ortegon, A.M.; Patel, S.; Punreddy, S.; Ge, P.; Sun, Y.; Lodish, H.F.; Stahl, A. Characterization of a heart-specific fatty acid transport protein. J. Biol. Chem 2003, 278, 16039–16044. [Google Scholar]
- Doege, H.; Baillie, R.A.; Ortegon, A.M.; Tsang, B.; Wu, Q.; Punreddy, S.; Hirsch, D.; Watson, N.; Gimeno, R.E.; Stahl, A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: Alterations in hepatic lipid homeostasis. Gastroenterology 2006, 130, 1245–1258. [Google Scholar]
- Zhou, W.; Madrid, P.; Fluitt, A.; Stahl, A.; Xie, X.S. Development and validation of a high-throughput screening assay for human long-chain fatty acid transport proteins 4 and 5. J. Biomol. Screen 2010, 15, 488–497. [Google Scholar]
- Herrmann, T.; van der Hoeven, F.; Grone, H.J.; Stewart, A.F.; Langbein, L.; Kaiser, I.; Liebisch, G.; Gosch, I.; Buchkremer, F.; Drobnik, W. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J. Cell Biol 2003, 161, 1105–1115. [Google Scholar]
- Herrmann, T.; Buchkremer, F.; Gosch, I.; Hall, A.M.; Bernlohr, D.A.; Stremmel, W. Mouse fatty acid transport protein 4 (FATP4): Characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene 2001, 270, 31–40. [Google Scholar]
- Lobo, S.; Wiczer, B.M.; Smith, A.J.; Hall, A.M.; Bernlohr, D.A. Fatty acid metabolism in adipocytes: Functional analysis of fatty acid transport proteins 1 and 4. J. Lipid Res 2007, 48, 609–620. [Google Scholar]
- Sobol, M.; Dahl, N.; Klar, J. FATP4 missense and nonsense mutations cause similar features in Ichthyosis Prematurity Syndrome. BMC Res. Notes 2011, 4. [Google Scholar] [CrossRef]
- Gertow, K.; Bellanda, M.; Eriksson, P.; Boquist, S.; Hamsten, A.; Sunnerhagen, M.; Fisher, R. Genetic and structural evaluation of fatty acid transport protein-4 in relation to markers of the insulin resistance syndrome. J. Clin. Endocrinol. Metab 2004, 89, 392–399. [Google Scholar]
- Doege, H.; Stahl, A. Protein-mediated fatty acid uptake: Novel insights from in vivo models. Physiology 2006, 21, 259–268. [Google Scholar]
- Gertow, K.; Rosell, M.; Sjogren, P.; Eriksson, P.; Vessby, B.; de Faire, U.; Hamsten, A.; Hellenius, M.L.; Fisher, R.M. Fatty acid handling protein expression in adipose tissue, fatty acid composition of adipose tissue and serum, and markers of insulin resistance. Eur. J. Clin. Nutr 2006, 60, 1406–1413. [Google Scholar]
- Bower, J.F.; Davis, J.M.; Hao, E.; Barakat, H.A. Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am. J. Physiol. Endocrinol. Metab 2006, 290, E87–E91. [Google Scholar]
- Fitscher, B.A.; Riedel, H.D.; Young, K.C.; Stremmel, W. Tissue distribution and cDNA cloning of a human fatty acid transport protein (hsFATP4). BBA Gene Struct. Expr 1998, 1443, 381–385. [Google Scholar]
- Schaffer, J.E.; Lodish, H.F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 1994, 79, 427–436. [Google Scholar]
- Stahl, A.; Hirsch, D.J.; Gimeno, R.E.; Punreddy, S.; Ge, P.; Watson, N.; Patel, S.; Kotler, M.; Raimondi, A.; Tartaglia, L.A. Identification of the major intestinal fatty acid transport protein. Mol. Cell 1999, 4, 299–308. [Google Scholar]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res 2009, 48, 52–61. [Google Scholar]
- Feng, A.J.; Wang, J.; Chen, D.F. Roles of fatty acid transport protein 4 in nonalcoholic fatty liver rats. Acta Acad. Med. Mil. Tertiae 2004, 26, 1726–1728. [Google Scholar]
- Schmuth, M.; Ortegon, A.M.; Mao-Qiang, M.; Elias, P.M.; Feingold, K.R.; Stahl, A. Differential expression of fatty acid transport protein in epidermis and skin appendages. J. Invest. Dermatol 2005, 6, 1174–1181. [Google Scholar]
- Cardon, L.R.; Abecasis, G.R. Using haplotype blocks to map human complex trait loci. Trends Genet 2003, 19, 135–140. [Google Scholar]
- Daly, M.J.; Rioux, J.D.; Schaffner, S.F.; Hudson, T.J.; Lander, E.S. High-resolution haplotype structure in the human genome. Nat. Genet 2001, 29, 229–232. [Google Scholar]
- Clark, A.G. The role of haplotypes in candidate gene studies. Genet. Epidemiol 2004, 27, 321–333. [Google Scholar]
- Stephens, M.; Smith, N.J.; Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet 2001, 68, 978–989. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Human BLAT Search. Available online: http://genome.ucsc.edu/cgi-bin/hgBlat?hgsid=168237115 accessed on 15 January 2012.
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res 2003, 13, 2129–2141. [Google Scholar]
- Thomas, P.D.; Kejariwal, A.; Guo, N.; Mi, H.; Campbell, M.J.; Muruganujan, A.; Lazareva-Ulitsky, B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis Uand coding SNP scoring tools. Nucleic Acids Res 2006, 34, W645–W650. [Google Scholar]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar]
- Kruglyak, L. Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat. Genet 1999, 22, 139–144. [Google Scholar]
| Tissue | LMW | BMW | SFT | AW | Liver | Brain | Heart | Small intestine |
|---|---|---|---|---|---|---|---|---|
| Expression level | 0.0101 ± 0.0163 b | 0.0011 ± 0.0163 b | 0.0009 ± 0.0163 b | 0.0012 ± 0.0163 b | 0.0010 ± 0.0163 b | 0.0014 ± 0.0163 b | 0.0005 ± 0.0163 b | 0.3494 ± 0.0167 a |
| Breed/Line | Traits 1 | g.5608778C>T genotype | p value | g.5608814G>A genotype | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| CC (n = 28) | CT (n = 82) | TT (n = 18) | GG (n = 36 ) | GA (n = 71) | AA (n = 21) | ||||
| S01 | BW (g) | 1807.85 (86.64) | 1925.18 (50.94) | 1720.00(108.06) | 0.1684 | 1925.83 (75.19) | 1779.57 (53.92) | 2077.14 (98.45) | 0.0229 |
| CW (g) | 1608.75 (80.21) | 1710.25 (47.75) | 1497.65(102.94) | 0.1397 | 1718.06 (69.43) | 1564.70 (50.89) | 1853.81 (90.91) | 0.0147 | |
| SEW (g) | 1508.93 (133.01) | 1597.16 (79.19) | 1827.06(170.70) | 0.3329 | 1611.11 (118.06) | 1573.66 (86.54) | 1716.67 (154.58) | 0.7223 | |
| EW (g) | 1252.14 (65.13) | 1330.51 (38.78) | 1160.53 (83.60) | 0.1747 | 1342.36 (56.57) | 1218.81 (41.47) | 1430.95 (74.07) | 0.0272 | |
| BMW (g) | 132.57 (7.06) | 104.59 (4.20) | 103.25 (9.34) | 0.0028 | 104.86 (6.50) | 111.77 (4.80) | 117.86 (8.51) | 0.4597 | |
| LMW (g) | 183.75 (11.54) | 150.57 (6.87) | 140.19 (15.26) | 0.0268 | 148.00 (10.39) | 156.44 (7.68) | 172.86 (13.61) | 0.3513 | |
| AW (g) | 46.75 (5.32) | 47.27 (3.17) | 42.75 (7.05) | 0.8426 | 50.17 (4.68) | 43.94 (3.46) | 48.62 (6.13) | 0.5287 | |
| p (HWE) | 0.001 | 0.1578 | |||||||
| Traits | CC (n = 43) | CT (n = 66) | TT (n = 27) | p value | GG (n = 45) | GA (n = 68) | AA (n = 23) | p value | |
| S05 | BW (g) | 1867.91 (65.66) | 2035.15 (53.00) | 2308.89 (82.86) | 0.0003 | 2171.56 (65.80) | 1917.35 (53.53) | 2125.22 (92.04) | 0.0077 |
| CW (g) | 1677.32 (60.84) | 1819.62 (49.11) | 2074.44(76.78) | 0.0004 | 1950.22 (60.86) | 1717.21 (49.51) | 1900.00 (85.12) | 0.0090 | |
| SEW (g) | 1574.53 (61.69) | 1691.97 (49.79) | 1940.74 (77.85) | 0.0015 | 1826.67 (61.55) | 1609.04 (50.07) | 1746.09 (86.10) | 0.0225 | |
| EW (g) | 1307.21 (49.86) | 1428.41 (40.25) | 1625.07 (62.92) | 0.0006 | 1532.11 (49.66) | 1338.49 (40.39) | 1495.65 (69.46) | 0.0072 | |
| BMW (g) | 104.42 (3.90) | 113.26 (3.15) | 122.67 (4.93) | 0.0156 | 116.56 (3.85) | 107.07 (3.13) | 119.61 (5.39) | 0.0575 | |
| LMW (g) | 137.40 (6.78) | 156.06 (5.48) | 173.22 (8.56) | 0.0047 | 163.71 (6.75) | 144.07 (5.49) | 161.78 (9.45) | 0.0532 | |
| AW (g) | 60.74 (10.04) | 59.74 (8.11) | 80.67 (12.67) | 0.3514 | 68.82 (9.87) | 63.36 (8.03) | 57.70 (13.82) | 0.7984 | |
| p (HWE) | 0.8539 | 0.7540 | |||||||
| Haplotype 1 (C-G) 2 | Haplotype 2 (C-A) 2 | Haplotype 3 (T-G) 2 | Haplotype 4 (T-A) 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Traits 1 | Regression Coefficient (SE) | p value | Regression Coefficient (SE) | p value | Regression Coefficient (SE) | p value | Regression Coefficient (SE) | p value |
| BW (g) | −115.324 (61.308) | 0.0621 | −196.131 (67.671) | 0.0044 | 185.277 (59.189) | 0.0021 | 140.408 (81.034) | 0.0854 |
| CW (g) | −99.764 (56.724) | 0.0809 | −180.269 (62.528) | 0.0046 | 167.779 (54.752) | 0.0026 | 122.698 (74.942) | 0.1039 |
| SEW (g) | −84.273 (57.175) | 0.1428 | −173.478 (62.974) | 0.0067 | 164.280 (55.099) | 0.0034 | 92.618 (75.611) | 0.2228 |
| EW (g) | −82.293 (46.354) | 0.0781 | −142.542 (51.211) | 0.0062 | 136.279 (44.773) | 0.0028 | 96.495 (61.301) | 0.1178 |
| BMW (g) | −7.240 (3.528) | 0.0421 | −5.351 (3.998) | 0.1830 | 7.466 (3.478) | 0.0336 | 6.649 (4.692) | 0.1588 |
| LMW (g) | −12.463 (6.190) | 0.0461 | −12.954 (6.968) | 0.0652 | 16.979 (6.027) | 0.0056 | 9.238 (8.250) | 0.2648 |
| AW (g) | −0.818 (9.006) | 0.9277 | −12.846 (10.054) | 0.2035 | 7.253 (8.868) | 0.4149 | 6.194 (11.868) | 0.6026 |
| Primer name | Primer sequence (5′→3′) | Annealing temperature (°C) | Product length (bp) | Amplified region |
|---|---|---|---|---|
| Primer pairs for measuring chicken FATP4 gene expression | ||||
| FATP4-F | CATCACCATCTCCAACTCCAAG | 61 | 126 | 1188–1313 |
| FATP4-R | GACTCAGGGCTTCCTTCTCCT | |||
| β-actin-F | GAGAAATTGTGCGTGACATCA | 60 | 180 | 685–836 |
| β-actin-R | CCTGAACCTCTCATTGCCA | |||
| Primer pairs for screening chicken FATP4 gene polymorphisms | ||||
| P-1F | TCCGGGATCCCACGAGAC | 54 | 243 | 5614016–5614358 |
| P-1R | ACGGCATTGGTGGCATAGCA | |||
| P-2F | ACGAGGCGGTTATTC | 55 | 309 | 5613698–5614006 |
| P-2R | GTCCCACCAGAGTCGCATTT | |||
| P-3F | CGCCGCGCTAGAAGT | 57 | 239 | 5613326–5613664 |
| P-3R | CCCGCTGGGAGCTGTAGT | |||
| P-4F | CAGGCCAAGATGCTGCGTCTGGCT | 55 | 215 | 5610456–5610670 |
| P-4R | ACACACCCCAGCGCACAGTT | |||
| P-5F | GTCCTGCTGCGGGTGAAGTG | 55 | 302 | 5609605–5609906 |
| P-5R | GAATTCACCAGGGCCGTCT | |||
| P-6F | CCGGTGCTCTTTCTCCATCT | 55 | 313 | 5608527–5608939 |
| P-6R | CTCTGCTGCTGAAGTCTGCC | |||
| P-7F | CGCTGCATGTGTGACCTTGT | 55 | 233 | 5608184–5608418 |
| P-7R | GCCATGCGGAAATACCTG | |||
| P-8F | GGCCCTGCTTCTGACAT | 55 | 152 | 5608087–5608238 |
| P-8R | GTCCCAAGGGCACACGTTAC | |||
| P-9F | GGGAACTCGGGGTACTGA | 55 | 245 | 5607424–5607668 |
| P-9R | GACAGACAGGCAGAACGAGT | |||
| P-10F | TTGCCCCTGCTAGATTGT | 55 | 204 | 5606940–5607143 |
| P-10R | AGGCTGCAGTTGCACTCGGT | |||
| P-11F | CATGGCGTGCGTTAAGAT | 55 | 166 | 5606652–5606817 |
| P-11R | AAGCCAATGGGGTACACT | |||
| P-12F | CCTTGGGCATGAGCGGTC AC | 55 | 141 | 5606311–5606451 |
| P-12R | TTGCTGGTGGCTGACTGATT | |||
| P-13F | GCTCCTCTCACACCTCGTT | 55 | 232 | 5605920–5606151 |
| P-13R | CCCTCCCCTCTCAGTTAC | |||
| P-14F | AGGGTGTCGCTGGTAAAC | 55 | 308 | 5605358–5605665 |
| P-14R | GTGCAGGAACCGTAGGA | |||
| P-15F | GAAGATGGAGCTGCGTAA | 55 | 247 | 5604728–5604974 |
| P-15R | CTAGTGTGCCTTTATACC | |||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Zhu, Q.; Yang, L.; Liu, Y.-P. Ontogenic Expression Pattern and Genetic Polymorphisms of the Fatty Acid Transport Protein 4 (FATP4) Gene in Chinese Chicken Populations. Int. J. Mol. Sci. 2012, 13, 6820-6835. https://doi.org/10.3390/ijms13066820
Wang Y, Zhu Q, Yang L, Liu Y-P. Ontogenic Expression Pattern and Genetic Polymorphisms of the Fatty Acid Transport Protein 4 (FATP4) Gene in Chinese Chicken Populations. International Journal of Molecular Sciences. 2012; 13(6):6820-6835. https://doi.org/10.3390/ijms13066820
Chicago/Turabian StyleWang, Yan, Qing Zhu, Ling Yang, and Yi-Ping Liu. 2012. "Ontogenic Expression Pattern and Genetic Polymorphisms of the Fatty Acid Transport Protein 4 (FATP4) Gene in Chinese Chicken Populations" International Journal of Molecular Sciences 13, no. 6: 6820-6835. https://doi.org/10.3390/ijms13066820




