Cancers 2021, 13(3), 509; https://doi.org/10.3390/cancers13030509 - 29 Jan 2021
Cited by 39 | Viewed by 7278
Abstract
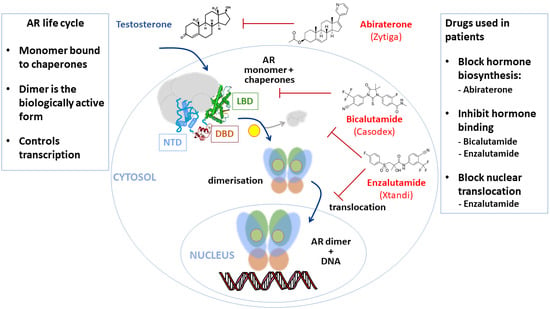
Prostate cancer (PCa) is the most common cancer in men in the West, other than skin cancer, accounting for over a quarter of cancer diagnoses in US men. In a seminal paper from 1941, Huggins and Hodges demonstrated that prostate tumours and metastatic
[...] Read more.
Prostate cancer (PCa) is the most common cancer in men in the West, other than skin cancer, accounting for over a quarter of cancer diagnoses in US men. In a seminal paper from 1941, Huggins and Hodges demonstrated that prostate tumours and metastatic disease were sensitive to the presence or absence of androgenic hormones. The first hormonal therapy for PCa was thus castration. In the subsequent eighty years, targeting the androgen signalling axis, where possible using drugs rather than surgery, has been a mainstay in the treatment of advanced and metastatic disease. Androgens signal via the androgen receptor, a ligand-activated transcription factor, which is the direct target of many such drugs. In this review we discuss the role of the androgen receptor in PCa and how the combination of structural information and functional screenings is continuing to be used for the discovery of new drug to switch off the receptor or modify its function in cancer cells.
Full article
(This article belongs to the Collection Prostate Cancer—from Molecular Mechanisms to Clinical Care)
►
Show Figures