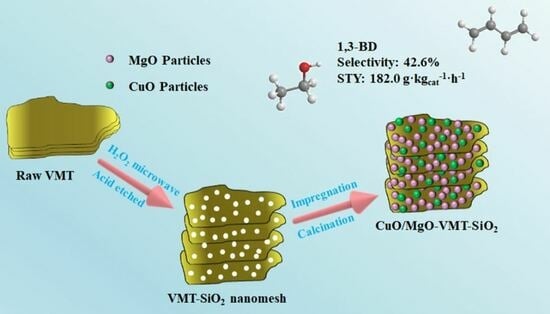
3.1. Textural Analysis
N
2 isothermal sorption curves and the textural properties of the samples are shown in
Figure 1 and
Table 1. According to
Figure 1a, it can be seen that all four samples exhibited IV-type isotherm adsorption curves and H4 adsorption hysteresis loops. The isotherms of the VMT sample are very close to the horizontal axis in the low-pressure region, and capillary condensation steps occur at a relatively high P/P
0 pressure range, characteristic of unconstrained single-layer or multi-layer adsorption in narrow pore channel structures [
8]. The adsorption curve of the VMT-SiO
2 sample deviates from the longitudinal axis at near-zero relative pressure, indicating that nitrogen has a strong interaction force in the microporous structures of the material. The obvious adsorption hysteresis loop occurred at around P/P
0 = 0.5, indicating the existence of abundant mesoporous structures due to the fact that most metal oxides impurities in vermiculite structure are dissolved by mixed acid and the remaining tetrahedral silica species form a mesoporous skeleton structure. When only MgO was loaded onto the VMT-SiO
2 support, the adsorption isotherm curve of MgO-VMT-SiO
2 became relatively gentle. When further loading CuO, the adsorption isotherm curve of CuO/MgO-VMT-SiO
2 became smoother, with only a slope higher than that of VMT, indicating that active components entered into the mesoporous channels of VMT-SiO
2, causing the plugging hole phenomenon to occur. The pore size distribution curves show that the pore sizes of the VMT-SiO
2-based samples gradually converged to less than 10 nm due to the final aggregation in mesopores with the loading of MgO and CuO active components. From
Figure 1b, it is clear that CuO/MgO-VMT-SiO
2 catalysts with different calcination temperatures show IV-type isothermal adsorption lines and H4 adsorption hysteresis loops at around P/P
0 = 0.5, which are characteristic of typical mesoporous materials. As the calcination temperature rises, the slope of the IV-type isothermal adsorption lines becomes more gentle with the smaller H4 adsorption hysteresis loops, which is probably due to the fact that the interaction between MgO, CuO and the carrier is enhanced and causes the partial destruction of some mesoporous structures. From the pore size distribution curve presented in
Figure 1, it can be observed that the most accessible pore sizes of the samples are mainly concentrated in 2–4 nm, and the calcination temperature had no significant influence on the pore size distribution.
Meanwhile, from the data concerning the textural properties of the samples (
Table 1), it is clear that after the mixed acid etching of VMT, the BET surface area and pore volume grew significantly from 12.5 m
2 g
−1 to 500.1 m
2 g
−1 and 0.08 cm
3 g
−1 to 0.43 cm
3 g
−1, respectively; however, the average pore size decreased significantly from 24.7 nm to 3.4 nm. When further loading a certain amount of MgO and CuO on VMT-SiO
2, the surface area and pore volume of the sample decreased sharply, but the average pore size increased. Notably, CuO/MgO-VMT-SiO
2 (500 °C) displayed the most diminished pore volume and BET surface area alongside the highest average pore size, suggesting that active MgO and CuO ingredients can better occupy the carrier pores.
3.2. SEM and TEM Images
Figure 2 shows the SEM and TEM images of the samples. We can observe the two-dimensional layered structure of VMT (see
Figure 2a). Upon being subjected to the mixed acid treatment, the nanomesh layer formed in the case of VMT-SiO
2 (see
Figure 2b). For the MgO-VMT-SiO
2 sample, it is evident that MgO is embedded in VMT-SiO
2, leading to slight sintering (
Figure 2c). Upon the loading of CuO, MgO and CuO were coated onto VMT-SiO
2 and formed particle clusters with different sizes (
Figure 2d–f). With increasing calcination temperature, more particle clusters can be obviously observed. This indicates that the interaction between MgO, CuO nanoparticles and VMT-SiO
2 outside the channel might be enhanced with increasing calcination temperature. The tiny MgO and CuO nanoparticles are more likely to migrate and aggregate on the surface to become larger particles, which is not conducive to the dispersion of MgO and CuO on the surface of VMT-SiO
2. Then, we preferably measured the lattice fringe spacing of CuO/MgO-VMT-SiO
2 (500 °C) by using the Fast Fourier Transform (FFT) method, as shown in the HRTEM image (
Figure 2g(g1,g2). The widths of the lattice fringes of CuO/MgO-VMT-SiO
2 (500 °C) are 2.91 Å and 2.32 Å, which represent the (310) MgSiO
3 plane and (111) CuO plane, respectively. This suggests that Mg-O-Si bonds formed over VMT-SiO
2 with the coexistence of MgSiO
3 and CuO crystals in the case of VMT-SiO
2. In order to determine the distribution of MgO and CuO in VMT-SiO
2, EDX-mapping analysis of the CuO/MgO-VMT-SiO
2 (500 °C) catalyst was carried out (
Figure 2h). It can be seen that the characteristic spectra of Mg and Cu elements are basically consistent with the spectra of Si and O elements in the VMT-SiO
2 skeleton, and the bright field and dark field are evenly distributed in the spectra. The MgO and CuO species are highly dispersed on the support in a cluster-like state.
3.3. Powder X-ray Diffraction
Figure 3 shows the powder X-ray diffraction patterns of the samples. The VMT-SiO
2 sample displays a sole prominent diffraction peak of silica at approximately 26.6° (see
Figure 3a), implying its high crystal phase purity. Moreover, the chemical composition of VMT-SiO
2 was determined through the use of X-ray fluorescence (XRF) spectroscopy, and the content of SiO
2 was found to be 99.8%. Upon loading MgO, the MgO-VMT-SiO
2 sample exhibits diffraction peaks at 42.8°, 62.2° and 28.1°, 30.7°, corresponding to the (200) and (220) crystal planes of MgO, and the (220) and (310) crystal planes of MgSiO
3. This suggests that the Mg element is present on the carrier surface as MgO and MgSiO
3. After introducing copper oxide, diffraction peaks of copper oxide appear at 35.5°, 38.71°, and 68.1°, which are assigned to the (11-1), (111), and (220) crystal planes, respectively. Meanwhile, the intensity of silica diffraction peak at 20–30° decreases, accompanied by increasing MgSiO
3 crystal phase. In
Figure 3b, the distinctive diffraction peaks of MgO, MgSiO
3, and CuO are displayed for all of the catalysts calcined at various temperatures. Among them, the sample calcined at 500 °C exhibits the most pronounced diffraction peak in terms of the MgSiO
3 crystal, and other diffraction characteristic peaks are very weak, indicating that the active components are more uniformly dispersed, forming strongly interacting Mg–O–Si species. Consequently, it can be presumed that the distribution of MgSiO
3 crystals and copper oxide crystals on VMT-SiO
2 can be modulated by altering the calcination temperature.
3.4. FT-IR Spectra
The FT-IR spectra of the samples are shown in
Figure 4. The VMT-SiO
2 sample exhibits an absorption peak at 970 cm
−1, corresponding to the vibration of Si–OH bonds [
9]. After loading MgO, this characteristic peak disappears. According to the literature, the surface hydroxyl groups of silica have a significant impact on the dispersion of the surface active components [
9,
10]. For MgO supported on silica materials, the weakening trend in the intensity of the Si-OH bond vibration bands indicated that the interaction between the loaded component and the carrier promotes the formation of Mg–O–Si bond structures. Therefore, for our present samples, the active hydroxyl groups on the surface of VMT-SiO
2 mainly anchor magnesium oxide during the preparation process. The disappearance of Si–OH bond vibration bands on the prepared catalyst implies that the loading of magnesium oxide results in a more uniform surface on the carrier, with MgO forming additional Mg–O–Si bond structures with Si–OH bonds. PXRD and TEM analyses both confirmed this phenomenon. Simultaneously, for the samples with different components, the infrared absorption peaks are observed at 772 cm
−1, 468 cm
−1, and 1073 cm
−1, which are consistent with the symmetric and antisymmetric stretching vibration of Si–O–Si bonds in the framework of VMT-SiO
2 [
11]. The infrared absorption peak at 1602 cm
−1 can be assigned to water physically adsorbed on the surface of the sample [
12,
13]. The incorporation of magnesium oxide appears to diminish the intensity of the Si–O–Si bond peak, indicating that magnesium oxide was successfully integrated into the VMT-SiO
2 framework and fostered robust interactions. Further adding copper oxide, the intensity of the Si–O–Si bond peak is largely weakened, suggesting that the inclusion of CuO serves to enhance the formation of magnesium–silicon oxidic composites. Finally, we investigated the impact of calcination temperatures on the catalyst structure. The catalysts calcined at three typical temperatures (450 °C, 500 °C, and 550 °C) display similar and obvious infrared absorption peaks at wavelengths of 1073 cm
−1, 970 cm
−1, 772 cm
−1, and 468 cm
−1. However, the peak intensity at 500 °C in these bands is notably weak, suggesting that a higher number of Si–O–Si bonds are being broken and stronger interactions are emerging between the active components over the samples. Furthermore, the characteristic absorption peaks across other wavenumbers exhibit similar patterns, demonstrating that the above three samples possess analogous structural characteristics.
3.6. Temperature Programmed Desorption
The Synthesis of 1,3-butadiene from ethanol requires the synergistic effect of various surface acid–base sites; therefore, it is necessary to detect the acidity and alkalinity of the catalyst.
Figure 6 shows the NH
3-TPD and CO
2-TPD curves of the catalysts, respectively. The NH
3-TPD curve (
Figure 6a) can be generally separated into three types of desorption peaks in different temperature ranges: a weak acid site from 100 °C to 200 °C, a medium–strong acid site from 200 °C to 300 °C, and a strong acid site above 300 °C. It can be observed that VMT-SiO
2 only contains weak acid sites. Upon loading MgO, the MgO-VMT-SiO
2 catalyst comprises weak, medium, and strong acid sites. The concentration of strong acid sites is the highest, followed by medium–strong and weak acid sites. When further loading CuO, the NH
3-TPD curves for the CuO/MgO-VMT-SiO
2 samples calcined at different calcination temperatures exhibit analogous peak shapes with respect to various acid sites, albeit with minor differences in peak areas. This suggests that altering the calcination temperature has a minimal impact on the acidity of the catalyst.
Concurrently, the CO
2-TPD curve (
Figure 6b) for the MgO/SiO
2 system can be categorized into three distinct desorption peaks: one is the hydroxyl group on the surface of magnesium oxide, namely Mg–OH (weak base); the second is a Mg–O pair (medium–strong base); and the third is the O
2− ion defect site (strong base) in magnesium oxide crystals. It can be seen that the VMT-SiO
2 has almost no basic sites. Upon loading MgO, the MgO-VMT-SiO
2 catalyst encompasses weak and medium–strong base sites, with a larger amount being noted in the latter than in the former. Notably, carbon dioxide desorption peaks were absent above 300 °C, suggesting that the catalyst lacked strong base sites. However, upon CuO loading, the peak patterns of the CO
2-TPD curve for each base site underwent significant alterations, leading to the emergence of a strong CO
2 desorption peak. It is noteworthy that the CuO/MgO-VMT-SiO
2 samples contain various types of basic sites regardless of the calcination temperature. As the temperature increases, the amounts of weak and strong base sites decrease, accompanied by increasing medium–strong base sites in relation to the samples. This suggests that the calcination temperature can be manipulated to adjust the alkaline properties of the catalyst. The data presented in
Table 2 and
Table 3 corroborate this trend, with changes in the content of various acid–base sites aligning with our previous analysis. The variances in acidity and alkalinity among the catalysts are primarily reflected in their total acidity and alkalinity. Specifically, the order of total acidity for the catalysts is as follows: CuO/MgO-VMT-SiO
2 (500 °C) < MgO-VMT-SiO
2 < CuO/MgO-VMT-SiO
2 (550 °C) < CuO/MgO-VMT-SiO
2 (450 °C) < VMT-SiO
2. On the other hand, the total basicity increases according to the sequence of VMT-SiO
2 < MgO-VMT-SiO
2 < CuO/MgO-VMT-SiO
2 (450 °C) < CuO/MgO-VMT-SiO
2 (500 °C) < CuO/MgO-VMT-SiO
2 (550 °C). Among the catalysts calcined at varying temperatures, CuO/MgO-VMT-SiO
2 (500 °C) exhibits the lowest total acid content, whereas CuO/MgO-VMTSiO
2 (450 °C) has the highest. Combined with PXRD analysis, it can be noted that the interaction between VMT-SiO
2 and MgO is the strongest at 500 °C, followed by 550 °C and 450 °C. As an alkaline earth metal, MgO counteracts some of the acidity produced by the sample during the interaction, resulting in a change in the above total acid content. As shown in the above-mentioned TEM and PXRD analyses, the interaction between MgO and CuO and VMT-SiO
2 enhanced with increasing temperature, and the introduction of CuO also promoted the formation of Mg-O-Si bonds in MgO. More Mg-O pairs (medium–strong base) led to an increase in the total base content of the catalyst with increasing calcination temperature.
3.7. Catalytic Performance
The catalytic performances of the catalysts obtained under the optimal reaction conditions are presented in
Table 4. The VMT-SiO
2 catalyst achieved an ethanol conversion of 26.4%, with the product predominantly consisting of ethylene. However, upon loading MgO, ethanol conversion decreased notably, and the main product was still ethylene despite some uncertain products. It has been demonstrated that the main rate-controlling step for the conversion of ethanol to 1,3-butadiene when using a magnesium–silicon catalyst is ethanol dehydrogenation [
14,
22]. Generally, ethanol dehydrogenation can occur in relation to materials containing redox sites or pure MgO [
23,
24]. When the MgO content is low, the activity of MgO for an ethanol dehydrogenation reaction is seriously insufficient, and no acetaldehyde is produced in the gas phase; therefore, it is unable to carry out the subsequent reactions. For the MgO-VMT-SiO
2 catalyst we prepared, MgO is embedded in VMT-SiO
2 (as shown in
Figure 2c), and a high calcination temperature also facilitates the formation of magnesium oxide clusters. These not only hinder the dispersion of MgO but also block the dehydration site on the surface of the carrier. As a consequence, the MgO-VMT-SiO
2 catalyst exhibited lower ethanol conversion. After loading copper oxide on the catalyst, a large amount of 1,3-butadiene began to be generated, and acetaldehyde selectivity was also greatly improved, with very low crotonaldehyde and crotyl alcohol formation. This indicates that the introduction of CuO inhibits the dehydration reaction of ethanol at the acidic site and increases additional redox sites, initiating the dehydrogenation of ethanol to acetaldehyde, followed by aldol condensation to crotonaldehyde, which is then reduced to crotyl alcohol by ethanol as the hydrogen donor. Finally, the crotyl alcohol is dehydrated to form 1,3-butadiene over the silanol acidic sites of the catalyst, as reported previously [
14].
To clarify the results presented above, we outlined the relationship curve between the catalytic performances and total acid–base content of the samples, as shown in
Figure 7. It can be observed that with increasing calcination temperatures in relation to CuO/MgO-VMT-SiO
2, the total acid/base ratio first decreases, reaching a minimum value at 500 °C, and then increases, reversing the trend of ethanol conversion and the selectivity of 1,3-butadiene. Namely, the larger the total acid/base ratio, the more difficult it is to generate the target products. At 500 °C, the total acid/base ratio of the CuO/MgO-VMT-SiO
2 catalyst is the smallest, which corresponds to higher ethanol conversion and 1,3-butadiene selectivity. This result indicates that we can regulate the production of 1,3-butadiene by changing the acid/base ratio on the surface of the catalyst.
From
Table 4, the investigated catalysts also produced a certain number of by-products (mainly containing butene, butanol, diethyl ether, and ethyl acetate). Specifically, the copper-containing catalysts exhibited relatively greater C
4 by-product formation (8–15.2% selectivity) at 325 °C. It has been reported that the reaction of butanol dehydration to produce 1,3-butadiene may be facilitated by Si–OH on the surface of SiO
2 [
14]. Consequently, the number of Si–OH on the SiO
2 surface impacts C
4 selectivity. The entire reaction process necessitates a good match between the Mg–O acid–base pair and adjacent Si–OH to generate 1,3-butadiene from crotonaldehyde. In our observations, we noted a strong interaction between Si–OH and MgO in the FT-IR spectrum, forming an Mg–O–Si bond structure. This led to the complete disappearance of the Si-OH bonds, indicating that the dehydration of the Si–OH (weak acid) site on the SiO
2 surface to form 1,3-butadiene is inhibited during the catalytic reaction process, thereby generating more C
4 by-products. As reported by Ordomsky et al., heavy components in the reaction process of ethanol to 1,3-butadiene are primarily catalyzed by strong base centers on the surface [
25]. When the alkalinity of the catalyst surface is excessively strong, it hinders the regulation of the condensation process of acetaldehyde, leading to the polycondensation of acetaldehyde. Combined with our analysis results, C
4 by-products contain some acetaldehyde polycondensation products, such as ethyl acetate; therefore, it is necessary to further regulate the balance of acid–base sites in the catalyst. According to
Table 2,
Table 3 and
Table 4, the quantity of acidic and strong basic sites is related to the degree of formation of ethylene and C
4 by-products, and the catalysts with the best performance are those containing a small number of acidic centers and a moderate proportion of medium–strong alkaline centers. In addition, the reaction steps of ethanol to 1,3-butadiene require these acid–base sites to achieve a subtle balance. Thus, these findings provide more insight into the connection between catalytic activity and the quantity and strength of acid–base sites.
Considering the best catalytic performance, which was achieved with the CuO/MgO-VMT-SiO
2 (500 °C) catalyst with an ethanol conversion rate of 30.7%, 1,3-butadiene selectivity of 42.6%, and STY
1,3-BD of 182.0 g·kg
cat−1·h
−1, as listed in
Table 4, we further investigated its catalytic performance by varying reaction temperatures, and those of the carrier VMT-SiO
2 sample were provided for comparison (see
Figure 8). For the VMT-SiO
2 sample (see
Figure 8a), when the temperature rose from 300 °C to 400 °C, ethanol conversion rose from 24.3% to 47.5%, while the selectivity of 1,3-butadiene declined from 6.1% to 0.3%. Specifically, when the temperature exceeded 350 °C, virtually no 1,3-butadiene was produced. The selectivity of ethylene progressively increased, contrary to that of acetaldehyde. For the CuO/MgO-VMT-SiO
2 (500 °C) sample (see
Figure 8b), it initially exhibited a high ethanol conversion of 47.8% at 300 °C. When the temperature rose to 350 °C, ethanol conversion decreased to the lowest value of 20.4% and then gradually rose with further increases in temperature. When changing the temperature, the selectivity and space-time yield of 1,3-butadiene first increased, reaching a maximum point at 325 °C, and then markedly reduced. The optimum temperature for this reaction is 325 °C, providing the highest 1,3-butadiene selectivity and space-time yield of 42.6% and 182.0 g·kg
cat−1·h
−1, respectively, as shown in
Table 4. According to the literature, in the ethanol to 1,3-butadiene MgO/SiO
2 catalytic system, the optimum acidic site corresponding to the NH
3 desorption temperature range is 200~300 °C, and the optimum alkaline site corresponding to the CO
2 desorption temperature range is 150~250 °C. Small molecules like ethylene desorb on magnesium silicate catalyst surface at elevated temperatures, and the ideal desorption temperature of ethylene ranges from 350 °C to 400 °C [
26]. This suggests that relatively low temperatures are more conducive to the production of 1,3-butadiene. Therefore, due to the relatively low reaction temperature, the undesorbed small olefin molecules polymerize on the catalyst surface to form carbonaceous components, covering the catalyst’s active center. Consequently, the conversion of ethanol declines with increasing temperature from 300 °C to 350 °C; the desorption of small molecules such as ethylene is blocked, and the selectivity of 1,3-butadiene increases. Above 350 °C, the desorbing degree of small olefin molecules on the catalyst surface increases, exposing more acid sites, resulting in a re-increase in ethanol conversion to 31.6%, an increase in ethylene content, and a decrease in 1,3-butadiene production. Given the close relationship between acetaldehyde and ethylene content and the formation of 1,3-butadiene, we also investigated the changing selectivity of acetaldehyde and ethylene with temperature in relation to the CuO/MgO-VMT-SiO
2 (500 °C) catalyst (see
Figure 8b). The selectivity of acetaldehyde initially increased with temperature, reaching a peak at 325 °C with a maximum selectivity of 26%, and then decreased with further increases in temperature. The selectivity of ethylene changed inversely in relation to that of acetaldehyde during this process. These findings suggest that alcohol dehydrogenation and dehydration are competitive reactions in relation to CuO/MgO-VMT-SiO
2 catalysts. The lower reaction temperature favored alcohol dehydrogenation, while the higher temperature facilitated alcohol dehydration.
Catalysts with excellent performance should not only have high catalytic activity and target product selectivity but also have good stability. In this regard, we compared the catalytic performance discovered in this study with results obtained from related works reported in the literature (See
Table 5). It can be seen from the table that WHSV is mostly around 1.0 h
−1, and the maximum STY of 1,3-butadiene is 0.48 g·g
cat−1·h
−1, with the minimum being 0.13 g·g
cat−1·h
−1. In this paper, the STY of 1,3-butadiene is still in the middle range under the lower reaction temperature and the highest WHSV reaction conditions, which indicates that we can further optimize the experimental conditions, reduce WHSV, and continue to improve catalytic performance. At the same time, we also studied the change in ethanol conversion and 1,3-butadiene selectivity of the best-performing catalyst within 36 h (see
Figure 9). It can be seen from the figure that within 36 h, ethanol conversion remained at about 30%, and the selectivity of 1,3-butadiene remained at about 40%, both of which had only slight fluctuations, indicating that the catalyst had good stability.