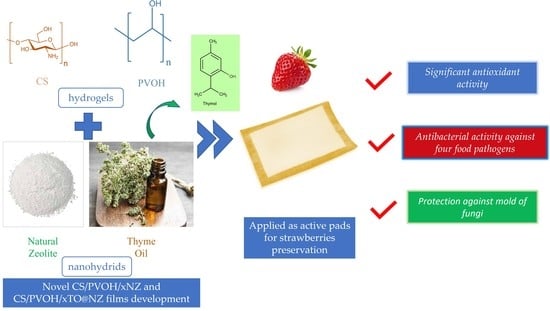
Thymol@Natural Zeolite Nanohybrids for Chitosan/Polyvinyl-Alcohol-Based Hydrogels Applied as Active Pads
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
2.2. FTIR Spectroscopy of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
2.3. Tensile Properties of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
2.4. Water–Oxygen Barrier Properties of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
2.5. Total Antioxidant Activity of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
2.6. Antibacterial Properties of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
2.7. Visual Evaluation of the Antimicrobial Activity of the Active Pads against Mold of Fungi on Strawberries
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Modification of NZ with Thymol
4.3. Preparation of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
4.4. XRD Analysis of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
4.5. FTIR Spectroscopy of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
4.6. Tensile Measurements of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
4.7. Water Vapor Transmission Rate Measurements and Water Diffusion Coefficient Calculation
4.8. Oxygen Transmission Rate Measurements and Oxygen Permeability Calculation
4.9. Total Antioxidant Activity of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
4.10. Antibacterial Activity Tests of CS/PVOH/xNZ and CS/PVOH/xTO@NZ Films
4.11. Packaging Test of CS/PVOH/HNT- and CS/PVOH/TO@HNT-Based Active Pads in Strawberry Protection against the Mold Fungi
4.12. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and Vegetable Waste Management and the Challenge of Fresh-Cut Salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Scarano, P.; Sciarrillo, R.; Tartaglia, M.; Zuzolo, D.; Guarino, C. Circular Economy and Secondary Raw Materials from Fruits as Sustainable Source for Recovery and Reuse. A Review. Trends Food Sci. Technol. 2022, 122, 157–170. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S. 11—Preservation of Fresh-Cut Fruits and Vegetables by Edible Coatings. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 225–242. ISBN 978-0-12-816184-5. [Google Scholar]
- Barrett, D.M.; Lloyd, B. Advanced Preservation Methods and Nutrient Retention in Fruits and Vegetables. J. Sci. Food Agric. 2012, 92, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wakisaka, M. A Review on the Modified Atmosphere Preservation of Fruits and Vegetables with Cutting-Edge Technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent Advances in Polysaccharide-Based Edible Coatings for Preservation of Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Park, H.J. Development of Advanced Edible Coatings for Fruits. Trends Food Sci. Technol. 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Trajkovska Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A.M. Chitosan Edible Films Enhanced with Pomegranate Peel Extract: Study on Physical, Biological, Thermal, and Barrier Properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=443&sort=GRN_No&order=DESC&startrow=1&type=basic&search=chitosan (accessed on 23 February 2023).
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent Advances in Polyvinyl Alcohol-Based Composite Films and Their Applications in Food Packaging. Food Packag. Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdulai, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich in Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of Clay-Polymer Nanocomposites Containing Bioactive Compounds for Food Packaging Applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Li, Q.; Ren, T.; Perkins, P.; Hu, X.; Wang, X. Applications of Halloysite Nanotubes in Food Packaging for Improving Film Performance and Food Preservation. Food Control 2021, 124, 107876. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The Effect of Different Preparation Methods on the Development of Chitosan/Thyme Oil/Montmorillonite Nanocomposite Active Packaging Films. J. Food Process. Preserv. 2019, 44, e14327. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Ansarifar, E.; Moradinezhad, F. Preservation of Strawberry Fruit Quality via the Use of Active Packaging with Encapsulated Thyme Essential Oil in Zein Nanofiber Film. Int. J. Food Sci. Technol. 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of Limonene Nano Coatings on Post-Harvest Shelf Life of Strawberries. LWT 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Nikolova, R.; Petkova, N.; Ivanov, I.; Lante, A. Biopreservation of Fresh Strawberries by Carboxymethyl Edible Coatings Enriched with a Bacteriocin from Bacillus Methylotrophicus BM47. Food Technol. Biotechnol. 2019, 57, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan–Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Pizato, S.; Vega-Herrera, S.S.; Chevalier, R.C.; Pinedo, R.A.; Cortez-Vega, W.R. Impact of Chitosan Coatings Enriched with Clove Essential Oil on Quality of Minimally Processed Strawberries. Braz. Arch. Biol. Technol. 2022, 65, 1–10. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, M.E.; Grande Tovar, C.D. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-Reinforced Electrospun Chitosan/PVA Nanocomposite Nanofibers for Biomedical Applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Aspanut, Z.; Majid, S.R.; Arof, A.K. FTIR Studies of Plasticized Poly(Vinyl Alcohol)–Chitosan Blend Doped with NH4NO3 Polymer Electrolyte Membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1068–1074. [Google Scholar] [CrossRef]
- Tvaruzkova, Z.; Bosáčekv, V. Characterization of hydroxyl groups of y zeolites by infrared spectra. Charact. Hydroxyl Groups Zeolites Infrared Spectra 1975, 29, 325–330. [Google Scholar]
- Ward, J.W. The Nature of Active Sites on Zeolites: III. The Alkali and Alkaline Earth Ion-Exchanged Forms. J. Catal. 1968, 10, 34–46. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Extruded Films of Blended Chitosan, Low Density Polyethylene and Ethylene Acrylic Acid. Carbohydr. Polym. 2013, 91, 666–674. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Matijasevic, D.; Niksic, M.; Rajic, N. Bactericidal Activity of Cu-, Zn-, and Ag-Containing Zeolites toward Escherichia Coli Isolates. Environ. Sci. Pollut. Res. 2017, 24, 20273–20281. [Google Scholar] [CrossRef]
- Pajnik, J.; Dikić, J.; Milovanovic, S.; Milosevic, M.; Jevtic, S.; Lukić, I. Zeolite/Chitosan/Gelatin Films: Preparation, Supercritical CO2 Processing, Characterization, and Bioactivity. Macromol. Mater. Eng. 2022, 307, 2200009. [Google Scholar] [CrossRef]
- Król, M.; Syguła-Cholewińska, J.; Sawoszczuk, T. Zeolite-Supported Aggregate as Potential Antimicrobial Agents in Gypsum Composites. Materials 2022, 15, 3305. [Google Scholar] [CrossRef]
- Pajnik, J.; Lukić, I.; Dikić, J.; Asanin, J.; Gordic, M.; Misic, D.; Zizović, I.; Korzeniowska, M. Application of Supercritical Solvent Impregnation for Production of Zeolite Modified Starch-Chitosan Polymers with Antibacterial Properties. Molecules 2020, 25, 4717. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Kalaycı, S.; Demirci, S.; Sahin, F. Determination of Antimicrobial Properties of Picaridin and DEET against a Broad Range of Microorganisms. World J. Microbiol. Biotechnol. 2014, 30, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kasperkowiak, M.; Strzemiecka, B.; Voelkel, A. Characteristics of Natural and Synthetic Molecular Sieves and Study of Their Interactions with Fragrance Compounds. Physicochem. Probl. Miner. Process. 2016, 52, 789–802. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]






| E | σuts | ε% | |
|---|---|---|---|
| CS/PVOH | 2249.3 ± 200.3 | 71.2 ± 1.8 c | 11.8 ± 0.9 |
| CS/PVOH/5NZ | 3064.3 ± 26.3 a | 89.0 ± 4.6 | 6.9 ± 1.1 e,f |
| CS/PVOH/10NZ | 2736.0 ± 351.3 b | 71.0 ± 16.7 c | 6.7 ± 2.8 f |
| CS/PVOH/15NZ | 2803.4 ± 345.3 b | 73.3 ± 4.5 c | 7.0 ± 2.1 e |
| CS/PVOH/5TO@NZ | 3304.0 ± 279.5 | 109.3 ± 17.2 | 6.8 ± 1.2 f |
| CS/PVOH/10TO@NZ | 3186.5 ± 125.2 | 103.7 ± 1.4 d | 6.7 ± 2.3 f |
| CS/PVOH/15TO@NZ | 3010.0 ± 481.3 a | 104.7 ± 5.5 d | 7.1 ± 2.2 e |
| Film Thickness (mm) | Water Vapor Transmission Rate (10−6 g/cm2·day) | Dw—Water Diffusion Coefficient (10−4 cm2/s) | Oxygen Transmission Rate (mL/m2·day) | PeO2 —Oxygen Permeability (10−7 cm2/s) | |
|---|---|---|---|---|---|
| CS/PVOH | 0.17 ± 0.012 | 1.06 ± 0.12 | 3.65 ± 0.11 | 38.2 ± 0.2 | 6.5 ± 0.3 |
| CS/PVOH/5NZ | 0.11 ± 0.010 a | 1.17 ± 0.10 | 3.21 ± 0.09 | 33.7 ± 0.2 | 3.7 ± 0.1 |
| CS/PVOH/10NZ | 0.12 ± 0.009 | 1.01 ± 0.13 | 2.67 ± 0.12 | 26.1 ± 0.2 | 2.6 ± 0.1 |
| CS/PVOH/15NZ | 0.10 ± 0.005 b | 0.92 ± 0.09 a | 2.33 ± 0.08 | 27.5 ± 0.3 | 2.2 ± 0.2 |
| CS/PVOH/5TO@NZ | 0.11 ± 0.006 a | 0.92 ± 0.07 a | 2.03 ± 0.06 | 15.5 ± 0.2 a | 1.8 ± 0.2 a |
| CS/PVOH/10TO@NZ | 0.08 ± 0.003 | 0.81 ± 0.07 b | 1.6 ± 0.05 | 34.4 ± 0.2 | 3.4 ± 0.1 |
| CS/PVOH/15TO@NZ | 0.10 ± 0.009 b | 0.79 ± 0.05 b | 1.8 ± 0.04 | 15.0 ± 0.2 a | 1.7 ± 0.2 a |
| Film Material | E. coli | S. aureus | S. enterica | L. monocytogenes |
|---|---|---|---|---|
| Inhibition (Diameter of Clear Zone) | Inhibition (Diameter of Clear Zone) | Inhibition (Diameter of Clear Zone) | Inhibition (Diameter of Clear Zone) | |
| CSPVOH | 3.57 ± 0.55 a | 4.23 ± 0.48 | 3.26 ± 0.17 a | 3.40 ± 0.70 a |
| CSPVOH5NZ | 0.00 b | 0.00 | 0.00 b | 0.00 b |
| CSPVOH10NZ | 0.00 b | 0.00 | 0.00 b | 0.00 b |
| CSPVOH15NZ | 0.00 b | 0.00 | 0.00 b | 0.00 b |
| CSPVOH5%TO@NZ | 3.93 ± 0.53 a | 4.73 ± 0.15 | 3.37 ± 0.16 a | 3.70 ± 0.14 a |
| CSPVOH10TO@NZ | 5.35 ± 0.30 | 5.32 ± 0.19 | 3.53 ± 0.18 c | 4.05 ± 0.18 |
| CSPVOH15TO@NZ | 8.35 ± 0.45 | 7.93 ± 0.54 | 3.55 ± 0.07 c | 4.48 ± 0.08 |
| Day 0 | Day 2 | Day 4 | Day 6 | |
| BLANK |  |  |  |  |
| CS/PVOH |  |  |  |  |
| CS/PVOH/15NZ |  |  |  |  |
| CS/PVOH/15TO@NZ |  |  |  |  |
| Day 7 | Day 9 | Day 10 | Day 12 | |
| CS/PVOH |  |  | ||
| CS/PVOH/15NZ |  |  | ||
| CS/PVOH/15TO@NZ |  |  |  |  |
| Day 14 | Day 16 | Day 18 | Day 21 | |
| CS/PVOH/15TO@NZ |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmas, C.E.; Kollia, E.; Avdylaj, L.; Kopsacheili, A.; Zaharioudakis, K.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Kehayias, G.; Karakassides, A.; et al. Thymol@Natural Zeolite Nanohybrids for Chitosan/Polyvinyl-Alcohol-Based Hydrogels Applied as Active Pads. Gels 2023, 9, 570. https://doi.org/10.3390/gels9070570
Salmas CE, Kollia E, Avdylaj L, Kopsacheili A, Zaharioudakis K, Georgopoulos S, Leontiou A, Katerinopoulou K, Kehayias G, Karakassides A, et al. Thymol@Natural Zeolite Nanohybrids for Chitosan/Polyvinyl-Alcohol-Based Hydrogels Applied as Active Pads. Gels. 2023; 9(7):570. https://doi.org/10.3390/gels9070570
Chicago/Turabian StyleSalmas, Constantinos E., Eleni Kollia, Learda Avdylaj, Anna Kopsacheili, Konstantinos Zaharioudakis, Stavros Georgopoulos, Areti Leontiou, Katerina Katerinopoulou, George Kehayias, Anastasios Karakassides, and et al. 2023. "Thymol@Natural Zeolite Nanohybrids for Chitosan/Polyvinyl-Alcohol-Based Hydrogels Applied as Active Pads" Gels 9, no. 7: 570. https://doi.org/10.3390/gels9070570