Enhanced Biocompatibility and Osteogenic Activity of Marine-Plankton-Derived Whitlockite Bone Granules through Bone Morphogenetic Protein 2 Incorporation
Abstract
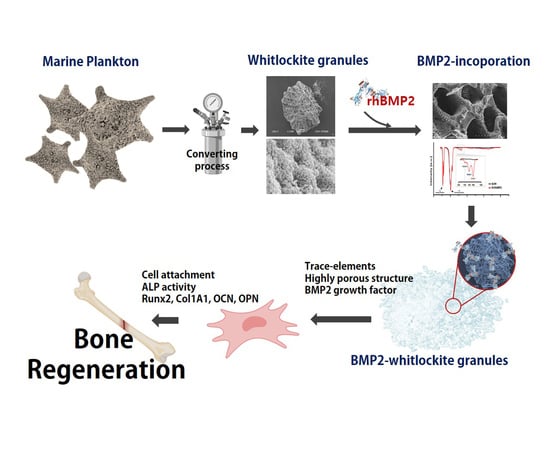
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porous WH Granules
2.2. Adsorption of BMP2 onto WH Granules
2.3. Physicochemical Analysis
2.4. Compressive Strength and Porosity
2.5. Cumulative Release of BMP2
2.6. hMSC Culture
2.7. Cell Culture on MP-WH /BMP2 Granules
2.8. Proliferation of hMSCs Cultured on MP-WH/BMP2 Granules
2.9. Cell Viability and Cytotoxicity
2.10. Observations of Cellular Adhesion to MP-WH and MP-WH/BMP2 Granules
2.11. Alkaline Phosphatase Activity
2.12. RT-PCR for Osteoblast-Related Gene Expression
2.13. Immunocytochemistry
2.14. Statistical Analysis
3. Results
3.1. Preparation of WH Bone Granules from Marine Plankton
3.2. BMP2-Incorporating WH Bone Granules
3.3. Characterization of BMP2-Incorporating MP-WH Granules
3.4. Compressive Strength and Porosity of MP-WH/BMP2 Granules
3.5. Cell Proliferation on MP-WH/BMP2 Granules
3.6. Cytotoxicity of MP-WH/BMP2 Granules
3.7. Cellular Adhesion to MP-WH/BMP2 Granules
3.8. Osteogenic Activity of MP-WH/BMP2 Granules
3.9. Phosphorylation of Smad 1/5/8 by MP-WH/BMP2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ. J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, D. X-ray crystallographic studies on apatites and calcified structures. Acta. Radiol. Suppl. 1955, 121, 1–59. [Google Scholar]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.K.; Hwang, N.S.; et al. In Vitro and In Vivo Evaluation of Whitlockite Biocompatibility: Comparative Study with Hydroxyapatite and β-Tricalcium Phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Subramanian, A.; James, R.; Harmon, M.D.; Yu, X.; Kumbar, S.G. Osteoinductive small molecules: Growth factor alternatives for bone tissue engineering. Curr. Pharm. Des. 2013, 19, 3420–3428. [Google Scholar] [CrossRef]
- Chin, M.; Ng, T.; Tom, W.K.; Carstens, M. Repair of alveolar clefts with recombinant human bone morphogenetic protein (rhBMP-2) in patients with clefts. J. Craniofac. Surg. 2005, 16, 778–789. [Google Scholar] [CrossRef]
- Notodihardjo, F.Z.; Kakudo, N.; Kushida, S.; Suzuki, K.; Kusumoto, K. Bone regeneration with BMP-2 and hydroxyapatite in critical-size calvarial defects in rats. J. Craniomaxillofac. Surg. 2012, 40, 287–291. [Google Scholar] [CrossRef]
- Jang, J.W.; Yun, J.H.; Lee, K.I.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Cho, K.S. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 113, 480–487. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Yang, S.S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, J.S.; Sung, H.M.; You, H.K.; Lee, J. Cellular attachment and osteoblast differentiation of mesenchymal stem cells on natural cuttlefish bone. J. Biomed. Mater. Res. A 2012, 100, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal stem cells: Mechanisms and role in bone regeneration. Postgrad. Med. J. 2014, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34, 1–10. [Google Scholar] [PubMed]
- Ignatius, A.A.; Schmidt, C.; Kaspar, D.; Claes, L.E. In vitro biocompatibility of resorbable experimental glass ceramics for bone substitutes. J. Biomed. Mater. Res. 2001, 55, 285–294. [Google Scholar] [CrossRef]
- Schopper, C.; Ziya-Ghazvini, F.; Goriwoda, W.; Moser, D.; Wanschitz, F.; Spassova, E.; Lagogiannis, G.; Auterith, A.; Ewers, R. HA/TCP compounding of a porous CaP biomaterial improves bone formation and scaffold degradation--a long-term histological study. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 458–467. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Zheng, G.B.; Cho, M.; Lee, J.H. Effect of Whitlockite as a new bone substitute for bone formation in spinal fusion and ectopic ossification animal model. Biomater. Res. 2021, 25, 34. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Bao, J.; Sun, X.; Zhang, X.; Zhang, X.; Ye, D.; Wei, J.; Liu, C.; Jiang, X.; et al. Trehalose Maintains Bioactivity and Promotes Sustained Release of BMP-2 from Lyophilized CDHA Scaffolds for Enhanced Osteogenesis In Vitro and In Vivo. PLoS ONE 2013, 8, e54645. [Google Scholar] [CrossRef]
- Wikesjo, U.M.; Guglielmoni, P.; Promsudthi, A.; Cho, K.S.; Trombelli, L.; Selvig, K.A.; Jin, L.; Wozney, J.M. Periodontal repair in dogs: Effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J. Clin. Periodontol. 1999, 26, 392–400. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.S.; Choi, K.H.; Jung, U.W.; Yun, J.H.; Choi, S.H.; Cho, K.S. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials 2010, 31, 3512–3519. [Google Scholar] [CrossRef]
- Kenley, R.; Marden, L.; Turek, T.; Jin, L.; Ron, E.; Hollinger, J.O. Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-2). J. Biomed. Mater. Res. 1994, 28, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Aghaloo, T.; Chou, Y.F.; Walder, B.; Zhang, X.; Soo, C.; Ting, K.; Wu, B. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007, 13, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Uijlenbroek, H.J.J.; Lin, X.; Zhang, X.; Deng, L.; Wismeijer, D.; Wang, M.; Wei, L.; Zheng, Y.; Liu, Y. Coralline Hydroxyapatite Coated with a Layer Biomimetic Calcium Phosphate Containing BMP-2 Induces Dose-Related Ectopic Bone Formation in Wistar Rats. Coatings 2021, 11, 1195. [Google Scholar] [CrossRef]
- Lin, X.; Hunziker, E.B.; Liu, T.; Hu, Q.; Liu, Y. Enhanced biocompatibility and improved osteogenesis of coralline hydroxyapatite modified by bone morphogenetic protein 2 incorporated into a biomimetic coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Boix, T.; Gómez-Morales, J.; Torrent-Burgués, J.; Monfort, A.; Puigdomènech, P.; Rodríguez-Clemente, R. Adsorption of recombinant human bone morphogenetic protein rhBMP-2m onto hydroxyapatite. J. Inorg. Biochem. 2005, 99, 1043–1050. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Pasciuta, R.; Grusovin, M.G.; Mancini, N.; Burioni, R. Evaluation of resistance against bacterial microleakage of a new conical implant-abutment connection versus conventional connections: An in vitro study. New Microbiol. 2016, 39, 49–56. [Google Scholar]
- Lucchese, A.; Matarese, G.; Manuelli, M.; Ciuffreda, C.; Bassani, L.; Isola, G.; Cordasco, G.; Gherlone, E. Reliability and efficacy of palifermin in prevention and management of oral mucositis in patients with acute lymphoblastic leukemia: A randomized, double-blind controlled clinical trial. Minerva. Stomatol. 2016, 65, 43–50. [Google Scholar]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. J. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Shah, A.K.; Lazatin, J.; Sinha, R.K.; Lennox, T.; Hickok, N.J.; Tuan, R.S. Mechanism of BMP-2 stimulated adhesion of osteoblastic cells to titanium alloy. Biol. Cell. 1999, 91, 131–142. [Google Scholar] [CrossRef]
- Kim, B.S.; Yang, S.S.; Kim, C.S. Incorporation of BMP-2 nanoparticles on the surface of a 3D-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surf. B. Biointerfaces 2018, 170, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Chien, K.B.; Aguado, B.A.; Shah, R.N. Osteogenic potential of BMP-2-releasing self-assembled membranes. Tissue Eng. Part A 2013, 19, 2664–2673. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar]
- Marie, P.J. Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 2008, 473, 98–105. [Google Scholar] [CrossRef]
- Kusuyama, J.; Bandow, K.; Ohnishi, T.; Hisadome, M.; Shima, K.; Semba, I.; Matsuguchi, T. Osteopontin inhibits osteoblast responsiveness through the down-regulation of focal adhesion kinase mediated by the induction of low-molecular weight protein tyrosine phosphatase. Mol. Biol. Cell 2017, 28, 1326–1336. [Google Scholar] [CrossRef]
- Jeong, B.C.; Kim, H.J.; Bae, I.H.; Lee, K.N.; Lee, K.Y.; Oh, W.M.; Kim, S.H.; Kang, I.C.; Lee, S.E.; Koh, G.Y.; et al. COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone 2010, 46, 479–486. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.W.; Kim, K.S.; Park, H.; Park, N.G.; Kim, B.-S. Enhanced Biocompatibility and Osteogenic Activity of Marine-Plankton-Derived Whitlockite Bone Granules through Bone Morphogenetic Protein 2 Incorporation. Bioengineering 2022, 9, 399. https://doi.org/10.3390/bioengineering9080399
Baek JW, Kim KS, Park H, Park NG, Kim B-S. Enhanced Biocompatibility and Osteogenic Activity of Marine-Plankton-Derived Whitlockite Bone Granules through Bone Morphogenetic Protein 2 Incorporation. Bioengineering. 2022; 9(8):399. https://doi.org/10.3390/bioengineering9080399
Chicago/Turabian StyleBaek, Ji Won, Ki Su Kim, Ho Park, Nak Gyu Park, and Beom-Su Kim. 2022. "Enhanced Biocompatibility and Osteogenic Activity of Marine-Plankton-Derived Whitlockite Bone Granules through Bone Morphogenetic Protein 2 Incorporation" Bioengineering 9, no. 8: 399. https://doi.org/10.3390/bioengineering9080399