Pulse Protein Isolates as Competitive Food Ingredients: Origin, Composition, Functionalities, and the State-of-the-Art Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Search Strategy
2.3. Software Used
3. Origins and Compositions of Pulse Crops
| Genus | Species | Common Name |
|---|---|---|
| Phaseolus | vulgaris | Common bean (Kidney, navy, great northern bean) |
| lunatus | Lima bean (Butter bean) | |
| coccineus | Runner bean (Scarlet runner) | |
| acutifolius | Tepary bean | |
| dumosus | Year bean | |
| Vigna | angularis | Adzuki bean |
| radiata | Mung bean (Green gram bean) | |
| mungo | Black gram bean | |
| aconitifolia | Mat bean, Moth bean | |
| unguiculata | Cowpea (Black-eyed pea) | |
| subterranea | Bambara bean (Earth pea) | |
| Lupinus | mutabilis | Lupin |
| albus | White lupin | |
| angustifolia | Blue lupin (Narrow-leafed lupin) | |
| luteus | Yellow lupin | |
| Pisum | sativum | Pea |
| Cicer | arietinum | Chickpea |
| Lens | culinaris | Lentil |
| Cajanus | cajan | Pigeon pea (Red gram bean) |
| Lablab | purpureus | Lablab bean (Hyacinth bean) |
| Canavalia | gladiate | Sword bean |
| Psophocarpus | tetragonolobus | Winged bean |
| Cyamopsis | tetragonoloba | Guar bean |
| Mucuna | pruriens | Velvet bean |
| Macrotyloma | uniflorum | Horse gram bean |
| Protein | Starch | Dietary Fibre | Fat | Ash | |
|---|---|---|---|---|---|
| Pea [22,25,26,27,35] | 14–31 | 30–50 | 3–20 | 1–4 | 2.3–3.7 |
| Chickpea [22,24,25,27,35] | 19–27 | 33.6–51.7 | 2.9–20.75 | 2–7 | 1.8–3.5 |
| Cowpea [24,25,27,29,35] | 24–28 | 33.1–63.6 | 10.06–34 | 1.26–2.22 | 2.9–4.4 |
| Pigeon pea [27,35] | 19.3–22.4 | NR | 6.4–7.25 | 2.74 | 0.04–2.13 |
| Lentil [22,25,27,35] | 23–31 | 37–59 | 7–30.5 | 1–3 | 2.1–3.2 |
| Lupin [22,25] | 32–44 | 1–9 | 14–55 | 5–15 | 2.6–3.9 |
| Faba bean [22,26,28,35] | 19–39 | 27–50 | 25–29.6 | 1.53–3.2 | 1.14–7.1 |
| Mung bean [23,24,35] | 14.6–32.6 | 29–58 | 3.8–6.15 | 0.17–7 | 0.17–5.87 |
| Common bean [25] | 17–27 | 31–43 | 18–30 | 1–5 | 3.2–5.2 |
4. Composition and Structure of Pulse Protein Isolates
4.1. Amino Acid Composition
4.2. Protein Fractions and Structures
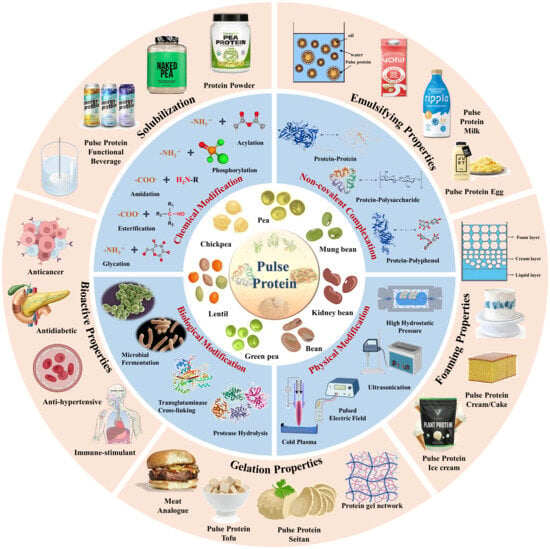
5. Functionality and Food Application of Pulse Protein Isolates
5.1. Solubility
5.2. Water/Oil Holding Capacity
5.3. Emulsifying and Foaming Properties
5.4. Gelation Properties
5.5. Bioactive Properties
5.6. Food Application
6. Modification Strategies of Pulse Protein Isolates
6.1. Chemical Covalent Modifications
6.2. Non-Covalent Complexation Modifications
6.3. Physical Modifications
- (1)
- Ultrasound induces cavitation and microstreaming currents, generating high temperatures and pressures for pulse protein modification, altering its spatial structure to enhance functionality. This includes heating and localized hydrodynamic shearing of protein molecules in a solution [18]. Many studies have demonstrated that when pulse protein, such as pea [106,107] and chickpea protein [59,108], was subjected to ultrasound treatment, it often leads to improved solubility and superior interfacial properties at both oil–water and gas–liquid interfaces.
- (2)
- Cold plasma constitutes the fourth state of matter, composed mainly of charged ions, free radicals, and electrons, which can induce protein modifications such as oxidation, cleavage, and polymerization, thus impacting protein structure [18]. Additionally, cold plasma modification can cause carbonylation and the cleaving of protein backbone peptide bonds. Bu et al. investigated the effect of cold plasma treatment on the structure and functionality of pea protein [109]. It was found that cold plasma modification increased the surface hydrophobicity of the protein and resulted in the formation of soluble aggregates through disulfide linkages. Altered protein secondary structures contribute to significant enhancements in gelation and emulsification properties [109].
- (3)
- A pulsed electric field (PEF) involves applying a strong electric field (>0.1 kV/cm) between two electrodes to a sample for a duration from milliseconds to nanoseconds [16]. Structural changes in pulse-treated proteins are driven by the response of charged chemical groups attempting to realign with the electric field through electrochemical reactions and polarization effects [110]. Numerous studies show that these external electrical fields can significantly alter both secondary and tertiary protein structures [111,112,113]. Chen et al. investigated the impact of a PEF on pea proteins and their binding capacity to EGCG through computer-based computational simulations [111]. As shown in Figure 8, PEF treatment (10 kV/cm) enhanced the binding affinity of pea protein isolates with EGCG, increasing the binding constant by 2.35 times and binding sites from 4 to 10 [111]. The number of amino acid residues involved in hydrophobic interactions in PEF-treated pea protein increased from 5 to 13.
- (4)
- High pressure modifies pulse protein through compression, disrupting noncovalent interactions, forming new non/semi-covalent bonds, and affecting factors like hydrogen bonds, electrostatic interactions, hydrophobic interactions, and semi-covalent bonds like disulfide bonds, ultimately shaping pulse protein conformation [18]. Hall et al. explored the effect of high-pressure modification on the structure and functionality of lentil, pea, and faba bean proteins, and 4 min pressure treatment (600 MPa, 5 °C) resulted in superior solubility, water-holding capacity, emulsifying, and foaming properties of pulse proteins [114]. Similarly, cowpea protein treated with high hydrostatic pressure (400 or 600 MPa) exhibited better gelation properties [115].
6.4. Biological Modifications
7. Conclusions and Future Research Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquez-Mota, C.C.; Rodriguez-Gaytan, C.; Adjibade, P.; Mazroui, R.; Galvez, A.; Granados, O.; Tovar, A.R.; Torres, N. The mTORC1-Signaling Pathway and Hepatic Polyribosome Profile Are Enhanced after the Recovery of a Protein Restricted Diet by a Combination of Soy or Black Bean with Corn Protein. Nutrients 2016, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.S.; Sun, J.Y.; Yin, Z.T.; Li, H.H.; Sun, X.T.; Zheng, F.P. Evaluation of antioxidant peptides generated from Jiuzao (residue after Baijiu distillation) protein hydrolysates and their effect of enhancing healthy value of Chinese Baijiu. J. Sci. Food Agric. 2020, 100, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.S.; Wang, R.; Yin, Z.T.; Sun, J.Y.; Wang, B.W.; Zhao, D.R.; Zeng, X.A.; Li, H.H.; Huang, M.Q.; Sun, B.G. Optimization of Jiuzao protein hydrolysis conditions and antioxidant activity in vivo of Jiuzao tetrapeptide Asp-Arg-Glu-Leu by elevating the Nrf2/Keap1-p38/PI3K-MafK signaling pathway. Food Funct. 2021, 12, 4808–4824. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.C.; Keoleian, G.A. Beyond Meat’s beyond Burger Life Cycle Assessment: A Detailed Comparison between a Plant-Based and an Animal-Based Protein Source; CSS Report; University of Michigan: Ann Arbor, MI, USA, 2018; pp. 1–38. [Google Scholar]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.; Greis, M.; Lu, J.K.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Improving soil health and human protein nutrition by pulses-based cropping systems. Adv. Agron. 2017, 145, 167–204. [Google Scholar]
- Zha, F.; Rao, J.; Chen, B. Modification of pulse proteins for improved functionality and flavor profile: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3036–3060. [Google Scholar] [CrossRef]
- Rivera, J.; Siliveru, K.; Li, Y. A comprehensive review on pulse protein fractionation and extraction: Processes, functionality, and food applications. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Reddy, P.P. Pulse Crops. In Nematode Diseases of Crops and Their Management; Springer: Berlin, Germany, 2021; pp. 67–95. [Google Scholar]
- Wang, R.; Li, L.L.; Feng, W.; Wang, T. Fabrication of hydrophilic composites by bridging the secondary structures between rice proteins and pea proteins toward enhanced nutritional properties. Food Funct. 2020, 11, 7446–7455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Wang, S.S.; Li, R.; Wang, Y.J.; Xiang, Q.S.; Li, K.; Bai, Y.H. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022, 124, 107351. [Google Scholar] [CrossRef]
- Teng, Y.X.; Zhang, T.; Dai, H.M.; Wang, Y.B.; Xu, J.T.; Zeng, X.A.; Li, B.; Zhu, X.W. Inducing the structural interplay of binary pulse protein complex to stimulate the solubilization of chickpea (Cicer arietinum L.) protein isolate. Food Chem. 2023, 407, 135136. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.C.; Easa, A.M.; Gammoh, S.; Kubow, S.; Alu’datt, M.H. Mechanisms of molecular and structural interactions between lentil and quinoa proteins in aqueous solutions induced by pH recycling. Int. J. Food Sci. Technol. 2022, 57, 2039–2050. [Google Scholar] [CrossRef]
- Fernando, S. Pulse protein ingredient modification. J. Sci. Food Agric. 2022, 102, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.; Gabriel, W.; Spallek, R.; Chang, Y.-C.; Mergner, J.; Wilhelm, M.; Bassermann, F.; Kuster, B. Linking post-translational modifications and protein turnover by site-resolved protein turnover profiling. Nat. Commun. 2022, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant proteins from green pea and chickpea: Extraction, fractionation, structural characterization and functional properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef]
- Dahiya, P.K.; Linnemann, A.R.; Van Boekel, M.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung Bean: Technological and Nutritional Potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. [Google Scholar] [CrossRef]
- Halimi, R.A.; Barkla, B.J.; Mayes, S.; King, G.J. The potential of the underutilized pulse bambara groundnut (Vigna subterranea (L.) Verdc.) for nutritional food security. J. Food Compos. Anal. 2019, 77, 47–59. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Robinson, G.H.J.; Balk, J.; Domoney, C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019, 44, 202–215. [Google Scholar] [CrossRef]
- Tang, X.; Shen, Y.T.; Zhang, Y.Q.; Schilling, M.W.; Li, Y.H. Parallel comparison of functional and physicochemical properties of common pulse proteins. LWT-Food Sci. Technol. 2021, 146, 111594. [Google Scholar] [CrossRef]
- Yan, M.; Guevara-Oquendo, V.H.; Rodriguez-Espinosa, M.E.; Yang, J.C.; Lardner, H.; Christensen, D.A.; Feng, X.; Yu, P.Q. Utilization of synchrotron-based and globar-sourced mid-infrared spectroscopy for faba nutritional research about molecular structural and nutritional interaction. Crit. Rev. Food Sci. Nutr. 2022, 62, 1453–1465. [Google Scholar] [CrossRef]
- Penchalaraju, M.; John Don Bosco, S. Legume protein concentrates from green gram, cowpea, and horse gram. J. Food Process. Preserv. 2022, 46, e16477. [Google Scholar] [CrossRef]
- Marinangeli, C.P.; Jones, P.J. Whole and fractionated yellow pea flours reduce fasting insulin and insulin resistance in hypercholesterolaemic and overweight human subjects. Br. J. Nutr. 2011, 105, 110–117. [Google Scholar] [CrossRef]
- Clark, J.L.; Taylor, C.G.; Zahradka, P. Rebelling against the (insulin) resistance: A review of the proposed insulin-sensitizing actions of soybeans, chickpeas, and their bioactive compounds. Nutrients 2018, 10, 434. [Google Scholar] [CrossRef]
- Reverri, E.J.; Randolph, J.M.; Steinberg, F.M.; Kappagoda, C.T.; Edirisinghe, I.; Burton-Freeman, B.M. Black beans, fiber, and antioxidant capacity pilot study: Examination of whole foods vs. functional components on postprandial metabolic, oxidative stress, and inflammation in adults with metabolic syndrome. Nutrients 2015, 7, 6139–6154. [Google Scholar] [CrossRef]
- Gowda, C.L.; Chaturvedi, S.; Gaur, P.; Sameer Kumar, C.; Jukanti, A. Pulses research and development strategies for India. In Pulses Handbook 2015; Commodity India: Bangalore, India, 2015; pp. 17–33. [Google Scholar]
- Hu, J.; Chen, G.; Zhang, Y.; Cui, B.; Yin, W.; Yu, X.; Zhu, Z.; Hu, Z. Anthocyanin composition and expression analysis of anthocyanin biosynthetic genes in kidney bean pod. Plant Physiol. Biochem. 2015, 97, 304–312. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Khan, F.N.; Bhardwaj, R.; Tripathi, K.; Bhardwaj, V.; Bhardwaj, R.; Gautam, R.K.; Kumar, A. Nutritional and Food Composition Survey of Major Pulses Toward Healthy, Sustainable, and Biofortified Diets. Front. Sustain. Food Syst. 2022, 6, 878269. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, M.; Zhu, D.; Zhang, J.; Wei, G. Metal-organic frameworks functionalized with nucleic acids and amino acids for structure-and function-specific applications: A tutorial review. Chem. Eng. J. 2022, 428, 131118. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Senevirathne, N.; Somaratne, G.; Bandara, N. Recent Trends in the Utilization of Pulse Protein in Food and Industrial Applications. ACS Food Sci. Technol. 2022, 2, 722–737. [Google Scholar] [CrossRef]
- Shrestha, S.; van’t Hag, L.; Haritos, V.S.; Dhital, S. Lentil and Mungbean protein isolates: Processing, functional properties, and potential food applications. Food Hydrocoll. 2022, 135, 108142. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Selvaraj, D.; Nile, A.S.; Ramalingam, S.; Kai, G.; Nile, S.H. Indian pulses: A review on nutritional, functional and biochemical properties with future perspectives. Trends Food Sci. Technol. 2019, 88, 228–242. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Sun, M.; Zhang, Y.; Fang, Y. Introducing panda bean (Vigna umbellata (Thunb.) Ohwi et Ohashi) protein isolate as an alternative source of legume protein: Physicochemical, functional and nutritional characteristics. Food Chem. 2022, 388, 133016. [Google Scholar] [CrossRef]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse proteins: From processing to structure-function relationships. Grain Legumes 2016, 3, 55–78. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Karaca, A.C.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Lu, Z.; He, J.; Zhang, Y.; Bing, D. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Huang, W.; Chen, L. Fabrication and characterization of lentil protein gels from fibrillar aggregates and the gelling mechanism study. Food Funct. 2020, 11, 10114–10125. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Hara-Hishimura, I.; Takeuchi, Y.; Inoue, K.; Nishimura, M. Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. Cell Mol. Biol. 1993, 4, 793–800. [Google Scholar] [CrossRef]
- Robin, A.; Kazir, M.; Sack, M.; Israel, A.; Frey, W.; Mueller, G.; Livney, Y.D.; Golberg, A. Functional Protein Concentrates Extracted from the Green Marine Macroalga Ulva sp., by High Voltage Pulsed Electric Fields and Mechanical Press. ACS Sustain. Chem. Eng. 2018, 6, 13696–13705. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Zhou, F.; Sun, H.; Cao, X.; Ye, Y.; Li, J. Detection of Lectin Protein Allergen of Kidney Beans (Phaseolus vulgaris L.) and Desensitization Food Processing Technology. J. Agric. Food Chem. 2021, 69, 14723–14741. [Google Scholar] [CrossRef]
- Schwenke, K.D.; Henning, T.; Dudek, S.; Dautzenberg, H.; Danilenko, A.N.; Kozhevnikov, G.O.; Braudo, E.E. Limited tryptic hydrolysis of pea legumin: Molecular mass and conformational stability of legumin-T. Int. J. Biol. Macromol. 2001, 28, 175–182. [Google Scholar] [CrossRef]
- Jain, A.; Kumar, A.; Salunke, D.M. Crystal structure of the vicilin from Solanum melongena reveals existence of different anionic ligands in structurally similar pockets. Sci. Rep. 2016, 6, 23600. [Google Scholar] [CrossRef]
- Mession, J.L.; Assifaoui, A.; Cayot, P.; Saurel, R. Effect of pea proteins extraction and vicilin/legumin fractionation on the phase behavior in admixture with alginate. Food Hydrocoll. 2012, 29, 335–346. [Google Scholar] [CrossRef]
- Shrestha, S.; van’t Hag, L.; Haritos, V.; Dhital, S. Comparative study on molecular and higher-order structures of legume seed protein isolates: Lentil, mungbean and yellow pea. Food Chem. 2023, 411, 135464. [Google Scholar] [CrossRef]
- Domoney, C.; Casey, R. Cloning and characterization of complementary DNA for convicilin, a major seed storage protein in Pisum sativum L. Planta 1983, 159, 446–453. [Google Scholar] [CrossRef]
- De Santis, M.A.; Rinaldi, M.; Menga, V.; Codianni, P.; Giuzio, L.; Fares, C.; Flagella, Z. Influence of Organic and Conventional Farming on Grain Yield and Protein Composition of Chickpea Genotypes. Agronomy 2021, 11, 191. [Google Scholar] [CrossRef]
- Zhu, Z.; Bassey, A.P.; Cao, Y.; Ma, Y.; Huang, M.; Yang, H. Food protein aggregation and its application. Food Res. Int. 2022, 160, 111725. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, Y.J.; Li, K.; Bai, Y.H.; Li, B.; Xu, W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT-Food Sci. Technol. 2020, 129, 109563. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Jing, X.; Chen, Z.J.; Wang, X.W. Effects of moderate-intensity pulsed electric field on the structure and physicochemical properties of foxtail millet (Setaria italica) prolamin. Cereal Chem. 2022, 100, 360–370. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Zannini, E.; Arendt, E.K. Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci. Technol. 2021, 110, 364–374. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, H.; Zeng, J.; Liu, Y.; Qin, Y.; Wang, M. Effect of subfreezing storage on the qualities of dough and bread containing pea protein. J. Sci. Food Agric. 2022, 102, 5378–5388. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-moisture meat analogues produced from yellow pea and faba bean protein isolates/concentrate: Effect of raw material composition and extrusion parameters on texture properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Kim, T.; Riaz, M.N.; Awika, J.; Teferra, T.F. The effect of cooling and rehydration methods in high moisture meat analogs with pulse proteins-peas, lentils, and faba beans. J. Food Sci. 2021, 86, 1322–1334. [Google Scholar] [CrossRef]
- Liu, F.F.; Li, Y.Q.; Wang, C.Y.; Zhao, X.Z.; Liang, Y.; He, J.X.; Mo, H.Z. Impact of pH on the physicochemical and rheological properties of mung bean (Vigna radiata L.) protein. Process Biochem. 2021, 111, 274–284. [Google Scholar] [CrossRef]
- Mohanan, A.; Harrison, K.; Cooper, D.M.L.; Nickerson, M.T.; Ghosh, S. Conversion of Pulse Protein Foam-Templated Oleogels into Oleofoams for Improved Baking Application. Foods 2022, 11, 2887. [Google Scholar] [CrossRef]
- Amagliani, L.; Silva, J.V.C.; Saffon, M.; Dombrowski, J. On the foaming properties of plant proteins: Current status and future opportunities. Trends Food Sci. Technol. 2021, 118, 261–272. [Google Scholar] [CrossRef]
- Shevkani, K.; Kaur, A.; Kumar, S.; Singh, N. Cowpea protein isolates: Functional properties and application in gluten-free rice muffins. LWT-Food Sci. Technol. 2015, 63, 927–933. [Google Scholar] [CrossRef]
- Toews, R.; Wang, N. Physicochemical and functional properties of protein concentrates from pulses. Food Res. Int. 2013, 52, 445–451. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. Effects of high hydrostatic pressure on the quality and functionality of protein isolates, concentrates, and hydrolysates derived from pulse legumes: A review. Trends Food Sci. Technol. 2021, 107, 466–479. [Google Scholar] [CrossRef]
- Al-Ali, H.A.; Shah, U.; Hackett, M.J.; Gulzar, M.; Karakyriakos, E.; Johnson, S.K. Technological strategies to improve gelation properties of legume proteins with the focus on lupin. Innov. Food Sci. Emerg. Technol. 2021, 68, 102634. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Patil, C.; Awasthi, A.; Paul, M. Antioxidative and antimicrobial properties of pulse proteins and their applications in gluten-free foods and sports nutrition. Innov. Food Sci. Emerg. Technol. 2022, 57, 5571–5584. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.R.; Osman, A.; Enan, G.; Abdel-Hameid, S.; Sitohy, M. Characterization and Antibacterial Activity of 7S and 11S Globulins Isolated from Cowpea Seed Protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef]
- Liu, Y.M.; Shahed-Al-Mahmud, M.; Chen, X.R.; Chen, T.H.; Liao, K.S.; Lo, J.M.; Wu, Y.M.; Ho, M.C.; Wu, C.Y.; Wong, C.H.; et al. A Carbohydrate-Binding Protein from the Edible Lablab Beans Effectively Blocks the Infections of Influenza Viruses and SARS-CoV-2. Cell Rep. 2020, 32, 108016. [Google Scholar] [CrossRef]
- Wang, W.B.; Li, Q.Q.; Wu, J.J.; Hu, Y.; Wu, G.; Yu, C.A.F.; Xu, K.W.; Liu, X.M.; Wang, Q.H.; Huang, W.J.; et al. Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg. Microbes Infect. 2021, 10, 1519–1529. [Google Scholar] [CrossRef]
- Li, S.Y.S.; Mejia, S.B.; Lytvyn, L.; Stewart, S.E.; Viguiliouk, E.; Ha, V.; de Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2017, 6, e006659. [Google Scholar] [CrossRef]
- Tak, Y.; Kaur, M.; Amarowicz, R.; Bhatia, S.; Gautam, C. Pulse derived bioactive peptides as novel nutraceuticals: A review. Int. J. Pept. Res. Ther. 2021, 27, 2057–2068. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Sun, H.; Wang, H.; Peng, W.; Zhou, Z.; Wang, H.; Pi, C.; Shi, Y.; He, X. Metabolism: A novel shared link between diabetes mellitus and Alzheimer’s disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef]
- Schouten, M.A.; Fryganas, C.; Tappi, S.; Romani, S.; Fogliano, V. Influence of lupin and chickpea flours on acrylamide formation and quality characteristics of biscuits. Food Chem. 2023, 402, 134221. [Google Scholar] [CrossRef]
- Mousa, M.M.H.; El-Magd, M.A.; Ghamry, H.I.; Alshahrani, M.Y.; El-Wakeil, N.H.M.; Hammad, E.M.; Asker, G.A.H. Pea peels as a value-added food ingredient for snack crackers and dry soup. Sci. Rep. 2021, 11, 22747. [Google Scholar] [CrossRef]
- Sinaki, N.Y.; Masatcioglu, M.T.; Paliwal, J.; Koksel, F. Development of Cellular High-Protein Foods: Third-Generation Yellow Pea and Red Lentil Puffed Snacks. Foods 2022, 11, 38. [Google Scholar] [CrossRef]
- Yang, M.; Li, N.N.; Tong, L.T.; Fan, B.; Wang, L.L.; Wang, F.Z.; Liu, L.Y. Comparison of physicochemical properties and volatile flavor compounds of pea protein and mung bean protein-based yogurt. LWT-Food Sci. Technol. 2021, 152, 112390. [Google Scholar] [CrossRef]
- Ramos-Diaz, J.M.; Kantanen, K.; Edelmann, J.M.; Jouppila, K.; Sontag-Strohm, T.; Piironen, V. Functionality of oat fiber concentrate and faba bean protein concentrate in plant-based substitutes for minced meat. Curr. Res. Food Sci. 2022, 5, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Zhang, S.C.; Zhao, X.Z.; Liu, Y.F.; Jiang, J.; Xiong, Y.L. Structural and rheological properties of mung bean protein emulsion as a liquid egg substitute: The effect of pH shifting and calcium. Food Hydrocoll. 2022, 126, 107485. [Google Scholar] [CrossRef]
- Shah, N.N.; Umesh, K.V.; Singhal, R.S. Hydrophobically modified pea proteins: Synthesis, characterization and evaluation as emulsifiers in eggless cake. J. Food Eng. 2019, 255, 15–23. [Google Scholar] [CrossRef]
- Charoensuk, D.; Brannan, R.G.; Chanasattru, W.; Chaiyasit, W. Physicochemical and emulsifying properties of mung bean protein isolate as influenced by succinylation. Int. J. Food Prop. 2018, 21, 1633–1645. [Google Scholar] [CrossRef]
- Caballero, S.; Davidov-Pardo, G. Comparison of legume and dairy proteins for the impact of Maillard conjugation on nanoemulsion formation, stability, and lutein color retention. Food Chem. 2021, 338, 128083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Huang, Y.; McClements, D.J.; Liu, X.B.; Wang, P.J.; Liu, F.G. Improving pea protein functionality by combining high-pressure homogenization with an ultrasound-assisted Maillard reaction. Food Hydrocoll. 2022, 126, 107441. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Yang, Y.; Zhang, L.; Li, J.; Wang, S. Functional properties and structural characteristics of phosphorylated pea protein isolate. Int. J. Food Sci. Technol. 2020, 55, 2002–2010. [Google Scholar] [CrossRef]
- Lin, D.Q.; Lu, W.; Kelly, A.L.; Zhang, L.T.; Zheng, B.D.; Miao, S. Interactions of vegetable proteins with other polymers: Structure-function relationships and applications in the food industry. Trends Food Sci. Technol. 2017, 68, 130–144. [Google Scholar] [CrossRef]
- Hao, L.L.; Sun, J.W.; Pei, M.Q.; Zhang, G.F.; Li, C.; Li, C.M.; Ma, X.K.; He, S.X.; Liu, L.B. Impact of non-covalent bound polyphenols on conformational, functional properties and in vitro digestibility of pea protein. Food Chem. 2022, 383, 132623. [Google Scholar] [CrossRef]
- Gunal-Koroglu, D.; Turan, S.; Capanoglu, E. Interaction of lentil protein and onion skin phenolics: Effects on functional properties of proteins and in vitro gastrointestinal digestibility. Food Chem. 2022, 372, 130892. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cui, F.Z.; McClements, D.J.; Xu, X.F.; Ma, C.C.; Wang, Y.T.; Liu, X.B.; Liu, F.G. Structural Characterization and Evaluation of Interfacial Properties of Pea Protein Isolate-EGCG Molecular Complexes. Foods 2022, 11, 2895. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.W.; Tang, Z.S.; Brennan, C.S.; Zeng, X.A. Thermomechanically micronized sugar beet pulp: Dissociation mechanism, physicochemical characteristics, and emulsifying properties. Food Res. Int. 2022, 160, 111675. [Google Scholar] [CrossRef] [PubMed]
- Parolia, S.; Maley, J.; Sammynaiken, R.; Green, R.; Nickerson, M.; Ghosh, S. Structure-Functionality of lentil protein-polyphenol conjugates. Food Chem. 2022, 367, 130603. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.G.; Li, Z.R.; Fan, X.; Liu, M.M.; Han, X.R.; Huang, J.R.; Xiong, Y.L.L. Multifaceted functionality of l-arginine in modulating the emulsifying properties of pea protein isolate and the oxidation stability of its emulsions. Food Funct. 2022, 13, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Liu, L.Y.; Li, Y.; Qian, H.F.; Tong, L.T.; Wang, L.L.; Zhou, X.R.; Wang, L.; Zhou, S.M. Effects of carboxymethylcellulose and soybean soluble polysaccharides on the stability of mung bean protein isolates in aqueous solution. LWT-Food Sci. Technol. 2020, 132, 109927. [Google Scholar] [CrossRef]
- Lin, J.W.; Yu, S.J.; Ai, C.; Zhang, T.; Guo, X.M. Emulsion stability of sugar beet pectin increased by genipin crosslinking. Food Hydrocoll. 2020, 101, 105459. [Google Scholar] [CrossRef]
- Kristensen, H.T.; Denon, Q.; Tavernier, I.; Gregersen, S.B.; Hammershoj, M.; Van der Meeren, P.; Dewettinck, K.; Dalsgaard, T.K. Improved food functional properties of pea protein isolate in blends and co-precipitates with whey protein isolate. Food Hydrocoll. 2021, 113, 106556. [Google Scholar] [CrossRef]
- Niu, D.B.; Ren, E.F.; Li, J.; Zeng, X.A.; Li, S.L. Effects of pulsed electric field-assisted treatment on the extraction, antioxidant activity and structure of naringin. Sep. Purif. Technol. 2021, 265, 118480. [Google Scholar] [CrossRef]
- Niu, D.B.; Zeng, X.A.; Ren, E.F.; Xu, F.Y.; Li, J.; Wang, M.S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef]
- Lian, F.; Sun, D.-W.; Cheng, J.-H.; Ma, J. Improving modification of structures and functionalities of food macromolecules by novel thermal technologies. Trends Food Sci. Technol. 2022, 129, 327–338. [Google Scholar] [CrossRef]
- Pan, J.Y.; Zhang, Z.L.; Mintah, B.K.; Xu, H.N.; Dabbour, M.; Cheng, Y.; Dai, C.H.; He, R.H.; Ma, H.L. Effects of nonthermal physical processing technologies on functional, structural properties and digestibility of food protein: A review. J. Food Process Eng. 2022, 45, e14010. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Sha, L. Comparative structural and emulsifying properties of ultrasound-treated pea (Pisum sativum L.) protein isolate and the legumin and vicilin fractions. Food Res. Int. 2022, 156, 111179. [Google Scholar]
- Sha, L.; Koosis, A.O.; Wang, Q.L.; True, A.D.; Xiong, Y.L. Interfacial dilatational and emulsifying properties of ultrasound-treated pea protein. Food Chem. 2021, 350, 129271. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Zhang, J.; Guo, X.B.; Lei, Y.D.; Yang, M. Effects of Ultrasonic Treatment on the Structure, Functional Properties of Chickpea Protein Isolate and Its Digestibility In Vitro. Foods 2022, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Feyzi, S.; Nayak, G.; Mao, Q.Q.; Kondeti, V.; Bruggeman, P.; Chen, C.; Ismail, B.P. Investigation of novel cold atmospheric plasma sources and their impact on the structural and functional characteristics of pea protein. Innov. Food Sci. Emerg. Technol. 2023, 83, 103248. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.H.; Wen, Q.H.; He, F.; Xu, F.Y.; Chen, B.R.; Zeng, X.A. Combination of pulsed electric field and pH shifting improves the solubility, emulsifying, foaming of commercial soy protein isolate. Food Hydrocoll. 2023, 134, 108049. [Google Scholar] [CrossRef]
- Chen, Z.L.; Li, Y.; Wang, J.H.; Wang, R.; Teng, Y.X.; Lin, J.W.; Zeng, X.A.; Woo, M.W.; Wang, L.; Han, Z. Pulsed electric field improves the EGCG binding ability of pea protein isolate unraveled by multi-spectroscopy and computer simulation. Int. J. Biol. Macromol. 2023, 244, 125082. [Google Scholar] [CrossRef]
- Melchior, S.; Calligaris, S.; Bisson, G.; Manzocco, L. Understanding the impact of moderate-intensity pulsed electric fields (MIPEF) on structural and functional characteristics of pea, rice and gluten concentrates. Food Bioprocess Technol. 2020, 13, 2145–2155. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhang, Y.F.; Yang, X.R.; Du, J.; Yu, D.Y.; Xie, F.Y. Effect of moderate electric fields on the structural and gelation properties of pea protein isolate. Innov. Food Sci. Emerg. Technol. 2022, 77, 102959. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Structure and function of pea, lentil and faba bean proteins treated by high pressure processing and heat treatment. LWT-Food Sci. Technol. 2021, 152, 112349. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Gelation of cowpea proteins induced by high hydrostatic pressure. Food Hydrocoll. 2021, 111, 106191. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Demand, V.; Kern, K.; Strube, A.; Szardenings, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Enzymatic Hydrolysis and Fermentation of Pea Protein Isolate and Its Effects on Antigenic Proteins, Functional Properties, and Sensory Profile. Foods 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno- functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Shuai, X.X.; Gao, L.Z.; Geng, Q.; Li, T.; He, X.M.; Chen, J.; Liu, C.M.; Dai, T.T. Effects of Moderate Enzymatic Hydrolysis on Structure and Functional Properties of Pea Protein. Foods 2022, 11, 2368. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Li, Y.Q.; Wang, C.Y.; Liang, Y.; Zhao, X.Z.; He, J.X.; Mo, H.Z. Physicochemical, functional and antioxidant properties of mung bean protein enzymatic hydrolysates. Food Chem. 2022, 393, 133397. [Google Scholar] [CrossRef]
- Wang, C.; Rao, J.; Li, X.; He, D.; Zhang, T.; Xu, J.; Chen, X.; Wang, L.; Yuan, Y.; Zhu, X. Chickpea protein hydrolysate as a novel plant-based cryoprotectant in frozen surimi: Insights into protein structure integrity and gelling behaviors. Food Res. Int. 2023, 169, 112871. [Google Scholar] [CrossRef]
- Bautista-Expósito, S.; Peñas, E.; Dueñas, M.; Silván, J.M.; Frias, J.; Martínez-Villaluenga, C. Individual contributions of Savinase and Lactobacillus plantarum to lentil functionalization during alkaline pH-controlled fermentation. Food Chem. 2018, 257, 341–349. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Li, Y. Pea protein composition, functionality, modification, and food applications: A review. Adv. Food Nutr. Res. 2022, 101, 71–127. [Google Scholar]
- Sun, X.D.; Arntfield, S.D. Gelation properties of salt-extracted pea protein isolate catalyzed by microbial transglutaminase cross-linking. Food Hydrocoll. 2011, 25, 25–31. [Google Scholar] [CrossRef]
- Zhan, F.C.; Tang, X.M.; Sobhy, R.; Li, B.; Chen, Y.J. Structural and rheology properties of pea protein isolate-stabilised emulsion gel: Effect of crosslinking with transglutaminase. Int. J. Food Sci. Technol. 2022, 57, 974–982. [Google Scholar] [CrossRef]
- Fang, L.Y.; Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Lin, J.J. Enhancing the Usability of Pea Protein Isolate in Food Applications through Modifying Its Structural and Sensory Properties via Deamidation by Glutaminase. J. Agric. Food Chem. 2020, 68, 1691–1697. [Google Scholar] [CrossRef] [PubMed]








| Amino Acid | Pea | Chickpea | Lentil | Mung Bean | Lupin | Cowpea | Faba Bean | Pigeon Pea |
|---|---|---|---|---|---|---|---|---|
| Essential AA | ||||||||
| Isoleucine (Ile, I) | 0.4–4.9 | 0.4–4.1 | 0.5–5.0 | 1.0–4.7 | 1.2–3.2 | 4.3–4.4 | 1.1–4.3 | 4.8 |
| Leucine (Leu, L) | 1.3–8.4 | 0.5–7.0 | 0.8–7.9 | 1.8–8.4 | 2.0–7.4 | 7.1–7.5 | 2.0–8.2 | 7.5 |
| Lysine (Lys, K) | 1.4–7.7 | 0.9–7.7 | 0.5–7.2 | 1.7–4.2 | 1.2–7.6 | 3.9–6.6 | 1.9 | 4.4 |
| Methionine (Met, M) | 0.2–3.3 | 0.1–1.9 | 0.1–2.9 | 0.3–1.9 | 0.2–0.3 | 1.2–1.3 | 0.2–0.8 | 1.2 |
| Phenylalanine (Phe, F) | 0.2–8.1 | 0.4–5.9 | 0.6–7.8 | 1.1–5.7 | 1.0–3.3 | 4.0–5.6 | 1.2 | 3.9 |
| Threonine (Thr, T) | 0.9–4.5 | 0.1–3.6 | 0.6–3.8 | 0.8–3.2 | 1.0–4.3 | 2.5–3.7 | 1.0–13.0 | 2.8 |
| Tryptophan (Trp, W) | 0.2–1.0 | 0.2–1.1 | 0.7–0.8 | 0.3–1.0 | 0.2–0.3 | 0.3–1.1 | 0.2–1.1 | NR |
| Valine (Val, V) | 0.4–5.2 | 0.4–3.8 | 0.7–5.3 | 1.2–5.2 | 1.1–3.5 | 4.6–4.9 | 1.2 | 4.7 |
| Arginine (Arg, R) | 1.2–8.7 | 0.5–10.3 | 0.9–7.8 | 1.7–6.3 | 2.8–10.9 | 7.3 | 2.6–10.3 | NR |
| Histidine (His, H) | 0.5–2.8 | 0.2–3.4 | 0.4–3.4 | 0.7–3.6 | 0.7–3.1 | 2.8–3.5 | 0.9–2.7 | 4.0 |
| Non-essential AA | ||||||||
| Alanine (Ala, A) | 0.8–4.8 | 0.3–4.8 | 2.0–4.2 | 3.5–4.4 | 0.9–2.8 | 3.7–4.3 | 1.2–4.2 | 4.5 |
| Aspartic acid (Asp, D) | 2.1–11.9 | 0.6–11.4 | 1.1–11.3 | 8.4–13.5 | 2.8–8.4 | 7.8–11.9 | 3.1 | 8.2 |
| Cystine (Cys, C) | 0.4–1.6 | 1.3–2.3 | 0.0–1.0 | 0.8–1.8 | 0.3–0.6 | 1.0–1.8 | 0.4–1.9 | 2.2 |
| Glutamic acid (Glu, E) | 2.9–18.5 | 1.7–17.3 | 2.4–15.1 | 6.1–21.7 | 6.2–26.1 | 6.0–18.5 | 4.6–13.0 | 6.2 |
| Glycine (Gly, G) | 0.8–4.8 | 0.3–4.1 | 1.0–4.8 | 4.1–4.26 | 1.0–3.7 | 4.1–4.2 | 1.2–4.2 | 4.6 |
| Proline (Pro, P) | 0.8–4.6 | 0.2–4.6 | 0.9–3.8 | 2.8–4.2 | 1.1–4.3 | 2.8–3.6 | 1.2–3.9 | 3.0 |
| Serine (Ser, S) | 0.8–5.7 | 0.1–4.9 | 1.1–4.9 | 2.5–5.0 | 1.3–6.0 | 2.6–5.6 | 1.3 | 2.7 |
| Tyrosine (Tyr, Y) | 0.6–3.8 | 0.2–3.7 | 0.5–3.2 | 3.3–3.4 | 1.0–4.3 | 3.2–5.0 | 0.9 | 3.2 |
| Albumin | Globulin | Glutelins | Prolamins | |
|---|---|---|---|---|
| Pea [43,45] | 18–25 | 55–65 | 3–4 | 4–5 |
| Chickpea [44] | 8–12 | 53–60 | 19–25 | 3–7 |
| Lentil [39,46] | 16–17 | 51–70 | 11 | 3–4 |
| Mung bean [23,39] | 16.3 | 62 | 13.3 | 0.9 |
| Faba bean [47] | 18.4–21.9 | 61.6–68 | 10.2–12.2 | 3.4–4.3 |
| Cowpea [25] | 4–12 | 58–80 | 10–15 | 1–3 |
| Lupin [25] | 9–22 | 44–60 | 6–23 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Li, X.; Liu, X.; Li, J.; Zeng, X.-A.; Li, Y.; Yuan, Y.; Teng, Y.-X. Pulse Protein Isolates as Competitive Food Ingredients: Origin, Composition, Functionalities, and the State-of-the-Art Manufacturing. Foods 2024, 13, 6. https://doi.org/10.3390/foods13010006
Zhu X, Li X, Liu X, Li J, Zeng X-A, Li Y, Yuan Y, Teng Y-X. Pulse Protein Isolates as Competitive Food Ingredients: Origin, Composition, Functionalities, and the State-of-the-Art Manufacturing. Foods. 2024; 13(1):6. https://doi.org/10.3390/foods13010006
Chicago/Turabian StyleZhu, Xiangwei, Xueyin Li, Xiangyu Liu, Jingfang Li, Xin-An Zeng, Yonghui Li, Yue Yuan, and Yong-Xin Teng. 2024. "Pulse Protein Isolates as Competitive Food Ingredients: Origin, Composition, Functionalities, and the State-of-the-Art Manufacturing" Foods 13, no. 1: 6. https://doi.org/10.3390/foods13010006