The Effects of Fluidized Bed Drying, Soaking, and Microwaving on the Phytic Acid Content, Protein Structure, and Digestibility of Dehulled Faba Beans
Abstract
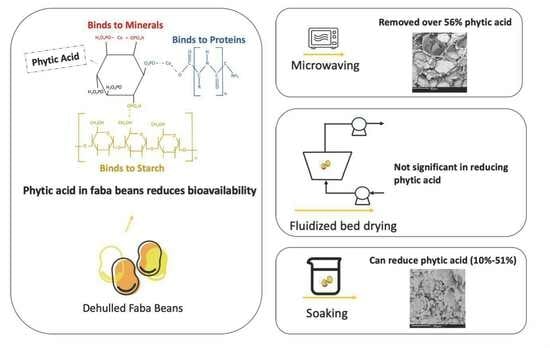
:1. Introduction
| Pulse | Processing Conditions | Phytic Acid Reduction (%) | References |
|---|---|---|---|
| Faba beans | Soaking | 32.7% | [23] |
| Lentils | Soaking in acidic condition | 37% | [15] |
| Indian tribal pulse | Soaking in alkaline condition | 11% | [14] |
| Lentils | Microwave + soaking | 45–52% | [15] |
| Green gram seed | Fluidized bed drying (50–70 °C) | Not significant | [11] |
2. Materials and Methods
2.1. Fluidized Bed Drying
2.2. Soaking
2.3. Microwaving
2.4. Phytic Acid Test
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. In Vitro Protein Digestibility
2.7. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Fluidized Bed Drying
3.1.1. Drying Curve
3.1.2. Phytic Acid
3.2. Soaking
3.3. Microwaving
3.4. Scanning Electron Microscopy
3.5. Protein Conformation Modification
3.6. In Vitro Protein Digestibility
3.7. Overall Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.-q.; Pulkkinen, M.; Wang, Y.-j.; Lampi, A.-M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- AEGIC. Australian Pulses: Quality, Versatility, Nutrition. 2017. Available online: https://www.aegic.org.au (accessed on 16 September 2023).
- Skylas, D.J.; Johnson, J.B.; Kalitsis, J.; Richard, S.; Whiteway, C.; Wesley, I.; Naiker, M.; Quail, K.J. Optimised dry processing of protein concentrates from Australian pulses: A comparative study of faba bean, yellow pea and red lentil seed material. Legume Sci. 2023, 5, e161. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; House, J.D. Factors influencing the quality of dietary proteins: Implications for pulses. Cereal Chem. 2017, 94, 49–57. [Google Scholar] [CrossRef]
- Akkad, R.; Kharraz, E.; Han, J.; House, J.D.; Curtis, J.M. The effect of short-term storage temperature on the key headspace volatile compounds observed in Canadian faba bean flour. Food Sci. Technol. Int. 2022, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U. Potential health benefits and problems associated with antinutrients in foods. Food Res. Int. 1993, 26, 131–149. [Google Scholar] [CrossRef]
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shivhare, U.S.; Vishwakarma, R.K. Anti-nutritional compounds in pulses: Implications and alleviation methods. Legume Sci. 2022, 4, e111. [Google Scholar] [CrossRef]
- Chen, H.; Diep, E.; Langrish, T.A.; Glasser, B.J. Continuous fluidized bed drying: Residence time distribution characterization and effluent moisture content prediction. AIChE J. 2020, 66, e16902. [Google Scholar] [CrossRef]
- Chen, H.; Rustagi, S.; Diep, E.; Langrish, T.A.; Glasser, B.J. Scale-up of fluidized bed drying: Impact of process and design parameters. Powder Technol. 2018, 339, 8–16. [Google Scholar] [CrossRef]
- Cheng, S.; Langrish, T.A. Fluidized bed drying of chickpeas: Developing a new drying schedule to reduce protein denaturation and remove trypsin inhibitors. J. Food Eng. 2023, 351, 111515. [Google Scholar] [CrossRef]
- Pande, R.; Mishra, H.N. Effect of fluidized bed heat treatment on insect mortality, proximate composition and antinutritional content of stored green gram (Vigna radiata) seeds. J. Food Chem. Nutr. 2013, 1, 94–99. [Google Scholar]
- Frias, J.; Vidal-Valverde, C.; Sotomayor, C.; Diaz-Pollan, C.; Urbano, G. Influence of processing on available carbohydrate content and antinutritional factors of chickpeas. Eur. Food Res. Technol. 2000, 210, 340–345. [Google Scholar] [CrossRef]
- Sarkhel, S.; Roy, A. Phytic acid and its reduction in pulse matrix: Structure–function relationship owing to bioavailability enhancement of micronutrients. J. Food Process Eng. 2022, 45, e14030. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Effect of various domestic processing methods on antinutrients and in vitro protein and starch digestibility of two indigenous varieties of Indian tribal pulse, Mucuna pruriens var. utilis. J. Agric. Food Chem. 2001, 49, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.R.; Zhong, F.; Anjum, F.M.; Khan, M.I.; Sharif, M.K.; Khan, M.; Haider, J.; Shah, F. Effect of soaking and microwave pretreatments on nutritional profile and cooking quality of different lentil cultivars. Pak. J. Food Sci 2014, 24, 186–194. [Google Scholar]
- Suhag, R.; Dhiman, A.; Deswal, G.; Thakur, D.; Sharanagat, V.S.; Kumar, K.; Kumar, V. Microwave processing: A way to reduce the anti-nutritional factors (ANFs) in food grains. LWT 2021, 150, 111960. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Singh, S.; Saxena, D.C. Physical, physicochemical and anti-nutritional properties of Horse Chestnut (Aesculus indica) seed. J. Food Meas. Charact. 2016, 10, 302–310. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Sharma, U.; Kumar, R.; Yadav, N. Effect of thermal processing on anti nutritional factors and in vitro bioavailability of minerals in desi and kabuli cultivars of chick pea grown in North India. Legume Res. Int. J. 2018, 41, 267–274. [Google Scholar] [CrossRef]
- Yang, H.W.; Hsu, C.K.; Yang, Y.F. Effect of thermal treatments on anti-nutritional factors and antioxidant capabilities in yellow soybeans and green-cotyledon small black soybeans. J. Sci. Food Agric. 2014, 94, 1794–1801. [Google Scholar] [CrossRef]
- Deng, Y.; Padilla-Zakour, O.; Zhao, Y.; Tao, S. Influences of high hydrostatic pressure, microwave heating, and boiling on chemical compositions, antinutritional factors, fatty acids, in vitro protein digestibility, and microstructure of buckwheat. Food Bioprocess Technol. 2015, 8, 2235–2245. [Google Scholar] [CrossRef]
- Irakli, M.; Lazaridou, A.; Biliaderis, C.G. Comparative evaluation of the nutritional, antinutritional, functional, and bioactivity attributes of rice bran stabilized by different heat treatments. Foods 2020, 10, 57. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 2018, 107, 660–668. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J.; Sotomayor, C.; Diaz-Pollan, C.; Fernandez, M.; Urbano, G. Nutrients and antinutritional factors in faba beans as affected by processing. Z. Leb.-Forsch. A 1998, 207, 140–145. [Google Scholar] [CrossRef]
- Keey, R.B. Introduction to Industrial Drying Operations; Pergamon: Oxford, UK, 1978; pp. 189–193. [Google Scholar]
- Haug, W.; Lantzsch, H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef]
- Hsu, H.; Vavak, D.; Satterlee, L.; Miller, G. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Aryee, A.N.; Boye, J.I. Improving the digestibility of lentil flours and protein isolate and characterization of their enzymatically prepared hydrolysates. Int. J. Food Prop. 2016, 19, 2649–2665. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legume Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Daneluti, A.L.M.; Matos, J.d.R. Study of thermal behavior of phytic acid. Braz. J. Pharm. Sci. 2013, 49, 275–283. [Google Scholar] [CrossRef]
- Oatway, L.; Vasanthan, T.; Helm, J.H. Phytic acid. Food Rev. Int. 2001, 17, 419–431. [Google Scholar] [CrossRef]
- Osman, A.M.A.; Hassan, A.B.; Osman, G.A.; Mohammed, N.; Rushdi, M.A.; Diab, E.E.; Babiker, E.E. Effects of gamma irradiation and/or cooking on nutritional quality of faba bean (Vicia faba L.) cultivars seeds. J. Food Sci. Technol. 2014, 51, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.A.; Curran, J.; Der, T. Effect of processing on antinutrient compounds in pulses. Cereal Chem. 2017, 94, 2–10. [Google Scholar] [CrossRef]
- Abd El-Hady, E.; Habiba, R. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci. Technol. 2003, 36, 285–293. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Makkar, H.; Becker, K. The effect of ionising radiation on antinutritional factors and the nutritional value of plant materials with reference to human and animal food. Food Chem. 2002, 78, 187–205. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J.; Estrella, I.; Gorospe, M.J.; Ruiz, R.; Bacon, J. Effect of processing on some antinutritional factors of lentils. J. Agric. Food Chem. 1994, 42, 2291–2295. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, X.; Fakayode, O.A.; Ma, H.; Zhou, C.; Xia, A.; Li, Q. Improving soaking efficiency of soybeans through sweeping frequency ultrasound assisted by parameters optimization. Ultrason. Sonochem. 2021, 79, 105794. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Feng, H.; Yin, Y.; Tang, J. Microwave drying of food and agricultural materials: Basics and heat and mass transfer modeling. Food Eng. Rev. 2012, 4, 89–106. [Google Scholar] [CrossRef]
- Terziev, N.; Daniel, G.; Torgovnikov, G.; Vinden, P. Effect of microwave treatment on the wood structure of Norway spruce and radiata pine. Bioresources 2020, 15, 5616–5626. [Google Scholar] [CrossRef]
- Hansson, L. Microwave Treatment of Wood. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2007. [Google Scholar]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, N.; Langrish, T.A. Comparative study of deteriorative changes in the ageing of milk powder. J. Food Eng. 2013, 114, 14–21. [Google Scholar] [CrossRef]
- Luo, Y.-W.; Xie, W.-H. Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia faba L.). CyTA J. Food 2013, 11, 43–49. [Google Scholar] [CrossRef]
- Kaspchak, E.; Mafra, L.I.; Mafra, M.R. Effect of heating and ionic strength on the interaction of bovine serum albumin and the antinutrients tannic and phytic acids, and its influence on in vitro protein digestibility. Food Chem. 2018, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sahu, C.; Panda, P.A.; Biswal, M.; Sah, R.P.; Lal, M.K.; Baig, M.J.; Swain, P.; Behera, L.; Chattopadhyay, K. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J. Sci. Food Agric. 2020, 100, 1598–1607. [Google Scholar] [CrossRef] [PubMed]






| Drying Time (min) | Moisture Content (%) | Phytic Acid (mg/100 g) | Reduction (%) |
|---|---|---|---|
| 5 | 40 | 770 ± 40 | ≤0 |
| 10 | 22 | 656 ± 24 | 8 ± 3 |
| 15 | 10 | 603 ± 16 | 15 ± 2 |
| 20 | 8 | 562 ± 64 | 21 ± 9 |
| Drying Time (min) | Moisture Content (%) | Phytic Acid (mg/100 g) | Reduction (%) |
|---|---|---|---|
| 5 | 31 | 572 ± 33 | 19 ± 5 |
| 10 | 14 | 655 ± 47 | 8 ± 7 |
| 15 | 8 | 548 ± 41 | 22 ± 6 |
| 20 | 4 | 814 ± 168 | ≤0 |
| Pre-Treatment | Moisture Content (%) | Phytic Acid (mg/100 g) | Reduction (%) |
|---|---|---|---|
| Water 12 h | 55 | 436 ± 75 | 39 ± 11 |
| Water 12 h 37 °C | 57 | 605 ± 68 | 15 ± 9 |
| Acid 12 h * | 56 | 538 ± 68 | 24 ± 10 |
| Acid 12 h 37 °C | 55 | 349 ± 75 | 51 ± 11 |
| Alkali 12 h ** | 54 | 552 ± 49 | 22 ± 7 |
| Alkali 12 h 37 °C | 55 | 640 ± 45 | 10 ± 6 |
| Pre-Treatment | Moisture Content (%) | Phytic Acid (mg/100 g) | Reduction (%) |
|---|---|---|---|
| Water 12 h + 30 s M | 48 | 271 ± 98 | 62 ± 14 |
| Water 12 h + 1 min M | 37 | 314 ± 112 | 56 ± 16 |
| Water 12 h + 2 min M | 13 | ≤0 | 100 ± 2 |
| Acid 12 h * + 30 s M | 52 | 506 ± 180 | 29 ± 25 |
| Acid 12 h * + 1 min M | 31 | 257 ± 85 | 64 ± 12 |
| Acid 12 h * + 2 min M | 6 | 29 ± 1 | 96 ± 1 |
| Alkali 12 h ** + 2 min M | 12 | 207 ± 9 | 71 ± 9 |
| Alkali 12 h (37 °C) ** + 2 min M | 16 | ≤0 | 100 ± 1 |
| Secondary Structure Component | Frequency (cm−1) | Raw Faba Beans (%) | FBD 120 °C_15 min (%) | FBD 140 °C_10 min (%) | Soaking for 12 h (%) | Microwaving for 2 min (%) |
|---|---|---|---|---|---|---|
| β-sheets | 1600–1638 | 42 | 46 | 58 | 1 | 79 |
| Unordered | 1638–1650 | 18 | 14 | 0 | 0 | 0 |
| α-helices | 1650–1660 | 15 | 11 | 21 | 1 | 0 |
| β-turns | 1660–1680 | 20 | 22 | 11 | 55 | 15 |
| Antiparallel β-sheets | 1680–1688 | 5 | 5 | 6 | 0 | 6 |
| β-type structures | 1690–1695 | 1 | 2 | 4 | 42 | 0 |
| σ (standard error, compared with raw faba bean) | 0.08 | 0.27 | 0.71 | 0.45 |
| Treatment | pH | Digestibility (%) | Increase (%) | |
|---|---|---|---|---|
| 1 | Fresh faba bean (Hulled) | 7.5 | 75.5 ± 0.5 | |
| 2 | Raw faba beans (Dehulled) | 6.8 | 87.4 ± 1.6 | 15.7 |
| 3 | FBD 140 °C for 10 min (Dehulled) | 6.8 | 88.0 ± 1.5 | 16.5 |
| 4 | FBD 120 °C for 15 min (Dehulled) | 6.8 | 88.0 ± 0.5 | 16.4 |
| 5 | Water Soaking for 12 h + Microwaving for 30 s (Dehulled) | 6.9 | 86.4 ± 0.7 | 14.4 |
| 6 | Water Soaking for 12 h + Microwaving for 2 min (Dehulled) | 6.8 | 88.3 ± 0.6 | 16.8 |
| 7 | Soaking for 12 h (Dehulled) | 7.0 | 84.9 ± 0.2 | 12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Skylas, D.J.; Whiteway, C.; Messina, V.; Langrish, T.A.G. The Effects of Fluidized Bed Drying, Soaking, and Microwaving on the Phytic Acid Content, Protein Structure, and Digestibility of Dehulled Faba Beans. Processes 2023, 11, 3401. https://doi.org/10.3390/pr11123401
Cheng S, Skylas DJ, Whiteway C, Messina V, Langrish TAG. The Effects of Fluidized Bed Drying, Soaking, and Microwaving on the Phytic Acid Content, Protein Structure, and Digestibility of Dehulled Faba Beans. Processes. 2023; 11(12):3401. https://doi.org/10.3390/pr11123401
Chicago/Turabian StyleCheng, Shu, Daniel J. Skylas, Chris Whiteway, Valeria Messina, and Timothy A. G. Langrish. 2023. "The Effects of Fluidized Bed Drying, Soaking, and Microwaving on the Phytic Acid Content, Protein Structure, and Digestibility of Dehulled Faba Beans" Processes 11, no. 12: 3401. https://doi.org/10.3390/pr11123401