1. Introduction
Diabetic retinopathy (DR) is a microvascular–neurodegenerative complication of diabetes mellitus (DM) and the leading cause of visual loss in working-age people across many countries [
1,
2]. Sustained hyperglycemia, multi-metabolic pathway alteration, and a variety of endogenous/exogenous risk factors induce oxidative stress (OS) as well as preclinical changes in the retinal neurovascular unit (NVU) [
3,
4,
5], as depicted in
Figure 1. A longer duration of DM, along with high blood pressure (HBP), augmented body mass index (BMI), and dyslipidemia, significantly increases the risk of developing DR [
3,
4,
6]. Outstanding strategies for DR management are emerging, including (1) innovative medical laser–surgical treatments, (2) chorio-retinal multimodal imaging, (3) electrophysiological probes of the retina and optic pathway, and (4) novel digital technology domains, including artificial intelligence (AI) and machine learning [
3,
6,
7,
8,
9,
10,
11].
Imaging and molecular-genetic biomarkers are directed to identify DR risk factors, to detail its clinical signs, to evaluate its progression, and to classify the stage of retinopathy [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Epidemiological and experimental studies have reported an important interindividual variation in DR development and severity [
18,
19]. Thus, identification of more precise biomarkers that may help to stratify the risk, or to evaluate the therapeutic response of DR, is mandatory.
Excessive generation of reactive oxygen species (ROS) and the impossibility of counteracting their downstream effects with appropriate activity of the antioxidant defenses (such as enzymatic scavengers: superoxide dismutase (SOD) and glutathione peroxidase (GPx)) induce deleterious damage to the nucleic acids, lipids, and proteins [
19,
20]. In this OS atmosphere, ROS can amplify specific cell signaling proteins and transcription factors, such as mitogen-activated protein kinase (MAPK), nuclear factor (erythroid-derived 2)-like 2 (Nrf2), nuclear factor kappa B (NF-kB)), protein kinase C (PKC), p53, and others, leading to the activation of angiogenic, autophagic, inflammatory, and apoptotic pathways, with detrimental consequences [
21,
22].
Apoptosis (AP), programmed cell death, is essential during central nervous system (CNS) development. Exhaustive research has been performed in recent years for elucidating the apoptotic core machinery and the molecular-genetic pathways involved in this process [
21,
22,
23,
24]. In fact, cells can survive or die by apoptosis depending on the balance between proapoptotic and antiapoptotic signals within and around them.
Figure 2 shows the intrinsic and extrinsic pathways of apoptosis. Each of these routes needs certain triggering signals to start an energy-dependent cascade of regulated molecular events. The proapoptotic mediators of the extrinsic (tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), Fas ligand (FasL), death-inducing signaling complex (DISC), Fas-associated death domain (FADD)-like IL-1β-converting enzyme-inhibitory protein (c-FLIP), TNFR1-associated death domain protein (TRADD), TNF-like weak inducer of apoptosis (TWEAK), and the nerve growth factor (NGF)) as well as the canonical intrinsic mitochondrial death (B-cell lymphoma 2 (Bcl-2) family proteins (group II and group III), proapoptotic homology (BH)3-only (including the following: Bcl2 modifying factor (Bmf), BIM, BID, Bcl-2 antagonist of cell death (BAD), p53 upregulated modulator of apoptosis (PUMA), and the phorbol-12-myristate-13-acetate-induced protein (NOXA)), Bcl-2-associated X protein (Bax), and the Bcl-2 homologous antagonist killer (Bak)) pathways, as well as the main antiapoptotic mediators from the intrinsic (Bcl-2 (group I), B-cell lymphoma-extra large (Bcl-XL)) and extrinsic (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein (c-FLIP) and nuclear factor kappa B (NF-κB)) pathways, finely orchestrate the balanced AP process and the switch between health and disease. It has been well established that AP is a key contribution to postembryonic development, to adult tissue homeostasis, and to the etiopathogenesis of multiple diseases, including cancer, neurodegenerative disorders, cardiovascular diseases, and ocular pathologies [
21,
22,
25,
26].
Small single-stranded noncoding microribonucleic acids (miRNAs/miRs) can regulate the expression of messenger RNAs and proteins within the cells and tissues in health and disease [
27]. miRNAs commonly act by repressing gene expression and by binding to the 3’ UTR fragment, inducing mRNA degradation. It is important to remember that the miRNAs not only target genes, but also specific and significant sections of signaling pathways. In this context, changes in miRNA expression levels have been reported to be associated with the development and progression of DR [
28], and these molecular actors have also been introduced as useful disease biomarkers. Furthermore, a crosslink between the expression levels of miRNAs and OS pathways has been widely investigated [
4,
29,
30].
Epigenetics encompasses all changes involving gene activity that do not implicate alterations in DNA sequence, such as DNA methylation, modification of histones, modifications of chromatin structure, loss of imprinting, and noncoding RNA activities [
31]. In this sense, the epigenome entails these genome modifications that do not affect the DNA sequence, selectively choosing, under specific conditions, whether genes will be switched “on” or “off” [
32]. It has been described that miRNAs contribute to a wide spectrum of disorders by means of epigenetic regulation of gene expression [
32,
33,
34]. In fact, epigenetic modifications are remarkably involved in T2DM [
35]. However, the human epigenome (in great proportion) remains unknown, creating an increasing interest in this regard in relation to DR research.
Because of this, evaluating the OS-related pathways, as well as the expression levels and biological functions of target miRNAs, is capital to understanding DR ethiopathogenesis. Therefore, we have taken into consideration that supramolecular targeted diagnosis, encompassing epigenetic modifications and signal transduction pathways, may play a more promising role in managing diabetic eyes.
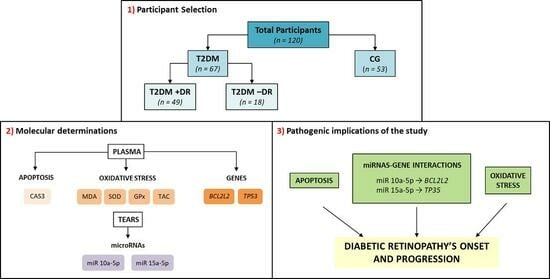
Therefore, the main purpose of this work is to report on the current level of knowledge on the clinical imaging and molecular-genetic landmarks that may help enlighten us to T2DM risk factors and pathogenic mechanisms to better understand the disease, and to elucidate potential applications for eye care in diabetics. By using ophthalmological imaging approaches (ocular biomicroscopy (BMC), ocular fundus examination (OF), retinography (RTG), optical coherence tomography (OCT)) and experimental tests (enzyme-linked immunosorbent assay (ELISA), next-generation sequencing (NGS), and real-time polymerase chain reaction (RT-PCR)), we registered a series of information to build a data network that may help in addressing new diagnostic challenges for DR in T2DM patients.
4. Discussion
We intended to shed light on the crosslink between OS and AP, as well as what epigenetic modifications may occur in DR patients. By collecting data from risk factors, clinical facts, retinal imaging, and pathogenic mechanisms, we built a data network that allowed us to obtain interesting results. We found that chronic hyperglycemia is responsible for OS status and its downstream effectors, which, in turn, induces a series of changes in signaling pathways, especially affecting the AP process. The OS biomarkers (MDA, SOD, GPx, TAC), the AP biomarkers (CAS3), the miRNAS 10a-5p and 15a-5b, and their predicted target genes BCL2L2 and TP53 have been identified as important players in DR in our study population. Characterizing these molecular actors and improving our knowledge about the crosstalk between them could open up some innovating paradigms with the main purpose of improving eye and vision care in diabetics.
Demographic and ophthalmologic characteristics of our cohort permitted us to detect that poor glycemic control, long-lasting T2DM, overweight/obesity, the presence of HBP and dyslipidemia, and familial history of DM were associated with a higher risk of DR and DR progression, confirming the growing body of evidence in recent reports [
1,
2,
3,
10,
11,
12,
13,
14,
52].
We performed bivariate correlation analyses to achieve greater knowledge of our clinical, retinal imaging, and biochemical analyses of our study participants, and our results reflected a significant lineal relation between the CST and CV (obtained via macular OCT examination) with the HbA1c values and the lipidic profile (Total Chol, LDL Chol, and VLDL Chol). Bjornstad et al. [
53] described the relationship between the retinal thickness and morphological changes on OCT from T2DM patients in the TODAY study, regarding the association between elevated HbA1c and increases in total retinal thickness. Also, Liang et al. [
54] reported that in T2DM patients with DR, the T Chol, LDL Chol, and HbA1c were closely correlated with CST. In a similar manner to us, Sasaki et al. [
55] described in a T2DM cohort that higher LDL Chol levels were associated with increased CST and CV in diabetics with mild DR without macular edema.
OS is known as the process in which the imbalance between the pro-oxidants and antioxidants (with higher proportion of pro-oxidative sources) damages cells, tissues, and organs. At a physiological level, ROS participate in the regulation of lysosomes and mitochondria. The mitochondria form ATP via the electron transport chain and oxidative phosphorylation, resulting in ROS production. However, a higher ROS level is harmful for cells and tissues, and the subsequent mitochondrial dysfunction severely damages the lysosomes. Therefore, under pathological conditions these systems fail and generate large amounts of pro-oxidants [
4,
13,
14,
18,
19,
28,
38,
43,
46,
56,
57,
58,
59]. Hyperglycemia leads over time to OS, which induces structural and functional damage to the retinal cell phenotypes, creating a harmful increased pro-oxidant and decreased antioxidant atmosphere [
4,
18,
19,
28,
60,
61,
62,
63].
In the present work, we found significantly higher levels of plasma MDA/TBARS and significantly lower plasma levels of SOD, GPx, and the TAC in T2DM vs. the CG, as is indicative of OS status. Other authors also reported similar findings to ours. Marsen-Bouterse et al. [
64], Domanico et al. [
65], Pan et al. [
66], Khan et al. [
67], and Wu et al. [
68], among many other authors worldwide [
4,
18,
19,
28,
59,
60,
61,
62,
63,
69], identified OS as a milestone in the onset and progression of DR. From a functional viewpoint, when cells are under stress, ROS can induce cell death via the extrinsic or intrinsic pathways (see
Figure 2). In this context, pathologic ROS overproduction induces AP cell death in the diabetic retina, as previously reported [
24,
25,
26,
69]. Regarding this, Xie et al. explored the role of mtDNA oxidative damage in high-glucose-induced dysfunction in human retinal vascular endothelial cells [
70].
Some relevant pathways induce upregulation of proapoptotic gene expression, and downregulation of neuroprotective factors. NF-κB is an important redox-sensitive transcription factor whose activation initiates a proapoptotic program. It has been recently reported that the signaling pathways of important transcription factors involved in OS, such as Nrf2, and inflammation, such as NF-κB, regulate the physiological redox status in the cells, subsequently modulating the cellular response to the above pathogenic mechanisms [
71]. As described herein, we identified specific AP molecules in biological samples from T2DM patients and healthy CG individuals. First, we looked for the expression of plasma CASP3, which is a cysteine–aspartyl protease encoded by the
CASP3 gene that crucially mediates the activation cascade of CAS, which, in turn, is responsible for programmed cell death (see
Figure 2). At the very onset of AP, CAS3 proteolytically cleaves the poly (ADP-ribose) polymerase (PARP) and initiates the sequence of facts leading to cell death. The most important PARP-1 function is DNA repair in response to a wide variety of exogenous/endogenous cellular stresses. In response to DNA damage, the activation of PARP-1 is an outstanding mechanism to keep cell homeostasis or to induce AP. The cleavage of PARP-1 by the above CAS leads to the generation of two fragments: (1) the 85-kD catalytic fragment and (2) the 24-kD DNA-binding domain. In this context, when comparing our results for the plasma samples from the T2DM+DR patients with those from their counterparts, significantly higher CAS3 expression was observed. The intrinsic apoptotic pathway is characterized by mitochondrial dysfunction and CAS activation (see
Figure 2). Jänicke et al. [
72] reported that CAS3 plays a pivotal role in the amplification of AP signaling via direct/indirect stimulation of the CAS downstream. CAS3 activation is a hallmark of AP and has been rendered a point of no return in the AP signaling molecular series. The AP of retinal cells has been pointed out as a preclinical sign of neurodegeneration in DR, as reported by Valverde et al. [
73]. These authors studied the neurodegeneration phenomenon that occurs early in the diabetic retina, concluding that disbalance between the intracellular signaling proapoptotic and survival factors was linked to the severity of damage in the retinal cell phenotypes in the course of DR.
Based on the OS and AP molecular data obtained in this population study, one can hypothesize that over time hyperglycemia induced the formation and accumulation of ROS (superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH)) that was reflected in a significant plasma MDA elevation and decreased SOD, GPx, and TAC in T2DM+DR patients. In addition, the increased OS core spread the oxidative damage which triggered the expression of transcription factors and target genes involved in AP, as exposed below.
We are trying to improve knowledge on the role of epigenetics in DR. As previously stated, epigenetics includes changes related to gene activity that do not involve DNA sequence alterations, such as DNA methylation, modification of histones, and noncoding RNAs, with the latter being important regulatory molecules (long-distance connectors and reprogramming factors) [
31,
32,
33,
34,
35]. In fact, several genes associated with oxidative stress in DR pathogenesis are influenced by specific epigenetic modifications [
74]. Maghbooli et al. [
75] studied 162 T2DM patients, and a relationship between genetic and epigenetic associations with the onset of DR was described. In spite of this, up to today the human epigenome (in great proportion) remains elusive. We studied the tear fluid expression levels and biological functions of miRNAs and target genes in DR ethiopathogenesis. Hence, the tear expression levels of miRNAs in T2DM patients +DR and −DR were assayed and compared with those from the CG. From all miRNAs (62) identified in tears from the study cohort (see
Table 6), 16 displayed differential signatures in diabetics versus the CG, with important contributions to several pathophysiological processes, such as OS, AP, cell cycle regulation, angiogenesis, and inflammation. We found that both miR-10a-5b and 15b-5p displayed differential signatures in the groups and subgroups of participants. These two miRNAs are highly involved in AP [
76]. The human gene that encodes miR-10a-5p is located upstream of the
HOXB4 gene on chromosome 17q21. The 5p means that the miRNA is from the 5’ arm of the hairpin. miR-10a-5p has been implicated in p53/stress response pathway regulation via its repression of a wide number of the key genes of the p53 network [
77]. In this sense, we have shown that miR-10a-5p was significantly downregulated in tears from the T2DM+DR patients, as compared with those from the CG. On the other hand, miR-15b-5p is encoded by the
MIR15B gene, located on 3q25.33, and widely involved in the pathogenic mechanisms of several conditions, such as Alzheimer’s and Parkinson’s diseases, cerebral stroke, and diabetic complications, as well as having a dual role (oncogenic and tumor suppressor) in cancer [
78]. In this work, we observed a noticeable upregulation of miR-15b-5p in the T2DM−DR and T2DM+DR subgroups when compared with the CG. Our findings characterize 10a-5p and 15b-5p miRNAs and their fingerprints in the regulatory network of DR in our T2DM patients. As ROS play relevant roles in a wide spectrum of intracellular signaling pathways by acting as second messengers, it may be hypothesized that in the presence of OS these miRNAs may intervene by targeting some nuclear proteins that control cell cycle, DNA repair, and AP, thus inhibiting the proliferation of the endothelial cells and inducing AP.
Because accumulating reports highlight pivotal roles for specific miRNAs in a variety of diabetic complications, we deal with studying the AP miR/target gene axis in T2DM patients with and without DR. In our study cohort, we saw that the
BCL2L2 gene and the
TP53 gene were upregulated in blood from T2DM+DR patients with respect to the CG (see
Figure 6) [
79,
80]. Therefore, our bioinformatic proceedings predicted that miR-10a-5p downregulation in tears was correlated with a decreased expression of their target genes, such as the
BCL2L2L and
TP53 genes, with regulatory AP functions. Also, the tear expression of miR-15b-5p was noticeably upregulated in the T2DM+DR patients, inhibiting the expression of their target genes, such as the
TP53 gene, thus blocking the AP process in diabetics. Therefore, differences in gene expression observed in this cohort can be understood as OS-related epigenetic modifications, regulated by a specific miRNA signature. We may propose that the miRNAs identified herein, and their target genes/signaling pathways, will help to achieve a better understanding of DR’s molecular and genetic bases.
Due to the enormous socioeconomic impact of DR [
81], it has to be considered that personalized medicine is a challenge for managing diabetic eyes and vision, being underpinned by larger scientific knowledge on the cellular and molecular-genetic basis of DM and its ocular complications. Our main goal is to improve current information about DR. In our cohort, the described clinical, molecular-genetic candidate biomarkers may potentially lead to ameliorating the diagnosis and to developing new therapies to improve eye and vision care in diabetics.
In an attempt to improve the prognosis of T2DM patients with and without DR, three potential therapeutic/prognostic approaches can be suggested: (1) avoiding or mitigating OS, (2) enhancing the cell survival pathways, and (3) blocking the AP cascade via CAS inhibitors and other biological strategies.
The present work has a series of strengths. DR diagnosis was performed by an ophthalmology specialist on the retina sections, which reinforces validity, and the collection of clinical and imaging data was performed via directly using the medical history, personal interview, and ophthalmological examination of each participant. Statistical processing was performed for the RE of each participant, taking into consideration that only initial NPDR cases were recruited for the present study. To our knowledge, miR-10a-5p, miR-15a-5b, and their target genes B2CL2L and TP53 were identified for the first time in relation to DR pathogenesis in the present work.
In contrast, some study limitations have also been considered by the research team. A relatively small sample size when classifying suitable study participants is present. Underestimation of DR cases is beyond our control. Some missing data were unrecoverable from the clinical histories and the personal interviews. It has to be considered when interpreting the results that an overstatement of the statistical power can appear in some data. Selection of candidate reference genes was performed via an extensive search of the literature and genes. RNA-seq-based assays will permit us to select and validate more appropriate candidates for future studies. Finally, we collected tears to evaluate the possibility of identifying miRNAs and their differential fingerprints in diabetic eyes (this is demonstrated herein). Due to the small number of tear samples, it was not possible to assay the predicted target genes, which were analyzed in blood samples. In the foreseeable future, we are planning to perform both assays on the two samples from each participant. Finally, we would like to state that the results of the qRT-PCR analysis of miR-15b-5p expression showed a noticeable trend toward upregulation in the diabetic groups, as compared with the CG. In addition, we found via qRT-PCR a slight downregulation of this miRNA in tears from the T2DM+DR when compared with the T2DM−DR.
In our opinion, pathological changes occurring in the choroid, retina, and vitreous can be reflected in the whole eye, including the ocular surface components, either via simple diffusion through the sclera or cornea, or via local and systemic circulation, and vice versa. Nevertheless, by analyzing tear film samples instead of the aqueous humor, vitreous, and/or blood for target molecules and gene concentrations, we can manage to create a very attractive promising window for the diagnosis of retinal diseases, as in the case of DR.
We suggest that supramolecular targeted diagnosis, as depicted herein, may play an important role in DR management. In this atmosphere, we propose that chronic hyperglycemia leads to OS and alteration of the retinal NVU, which in the presence of concrete risk factors induces relevant changes in a wide variety of molecules that we have identified in tears and plasma samples, such as MDA, SOD, GPx, CAS3, miR-10a-5p, miR-15b-5p, and the BCL2L2 and TP53 genes.