Semen Cryopreservation to Expand Male Fertility in Cancer Patients: Intracase Evaluation of Semen Quality
Abstract
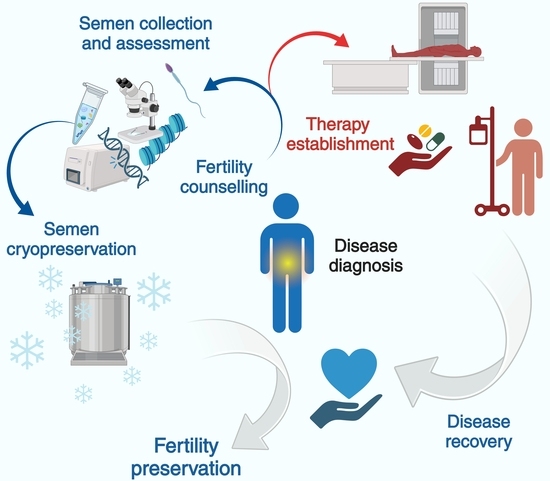
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Semen Quality Assessment
2.2.1. Motility Assessment
2.2.2. Morphology Assessment
2.3. Pre-Freezing and Freezing Procedures
2.4. Statistical Analysis
3. Results
3.1. Epidemiological Data and Clinical Characteristics
3.2. Semen Quality Assessment in Cancer Subgroups
3.3. Comparison and Correlation between Pre- and Post-Cryopreservation Semen Parameters
3.4. Age-Dependent Impact on Semen Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchiani, S.; Degl’Innocenti, S.; Dabizzi, S.; Tamburrino, L.; Fino, M.G.; Traini, G.; Calamai, C.; Maggi, M.; Vignozzi, L.; Baldi, E.; et al. Semen Cryopreservation for Men Banking for Oligozoospermia, Cancers, and Other Conditions: 24 Years’ Experience of an Italian Bank. J. Clin. Med. 2023, 12, 4657. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Finelli, R.; Baskaran, S.; Agarwal, A. Dysregulation of Key Proteins Associated with Sperm Motility and Fertility Potential in Cancer Patients. Int. J. Mol. Sci. 2020, 21, 6754. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, S.; Filimberti, E.; Magini, A.; Krausz, C.; Lombardi, G.; Fino, M.G.; Rastrelli, G.; Maggi, M.; Baldi, E. Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: Prediction of post-thaw outcome using basal semen quality. Fertil. Steril. 2013, 100, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, L.; Traini, G.; Marcellini, A.; Vignozzi, L.; Baldi, E.; Marchiani, S. Cryopreservation of Human Spermatozoa: Functional, Molecular and Clinical Aspects. Int. J. Mol. Sci. 2023, 24, 4656. [Google Scholar] [CrossRef]
- Patel, P.; Kohn, T.P.; Cohen, J.; Shiff, B.; Kohn, J.; Ramasamy, R. Evaluation of Reported Fertility Preservation Counseling Before Chemotherapy Using the Quality Oncology Practice Initiative Survey. JAMA Netw. Open 2020, 3, e2010806. [Google Scholar] [CrossRef]
- Amirjannati, N.; Sadeghi, M.; Hosseini Jadda, S.H.; Ranjbar, F.; Kamali, K.; Akhondi, M.A. Evaluation of semen quality in patients with malignancies referred for sperm banking before cancer treatment. Andrologia 2011, 43, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Bizet, P.; Saias-Magnan, J.; Jouve, E.; Grillo, J.M.; Karsenty, G.; Metzler-Guillemain, C.; Perrin, J. Sperm cryopreservation before cancer treatment: A 15-year monocentric experience. Reprod. Biomed. Online 2012, 24, 321–330. [Google Scholar] [CrossRef]
- Bonetti, T.C.; Pasqualotto, F.F.; Queiroz, P.; Iaconelli, A., Jr.; Borges, E., Jr. Sperm banking for male cancer patients: Social and semen profiles. Int. Braz. J. Urol. 2009, 35, 190–197, discussion 197–198. [Google Scholar] [CrossRef]
- Shrem, G.; Azani, L.; Feferkorn, I.; Listovsky, T.; Hussaini, S.; Farber, B.; Dahan, M.H.; Salmon-Divon, M. Effect of Malignancy on Semen Parameters. Life 2022, 12, 922. [Google Scholar] [CrossRef]
- Williams, D.H.t.; Karpman, E.; Sander, J.C.; Spiess, P.E.; Pisters, L.L.; Lipshultz, L.I. Pretreatment semen parameters in men with cancer. J. Urol. 2009, 181, 736–740. [Google Scholar] [CrossRef]
- Crawshaw, M.A.; Glaser, A.W.; Hale, J.P.; Sloper, P. Young males’ experiences of sperm banking following a cancer diagnosis-a qualitative study. Hum. Fertil. 2008, 11, 238–245. [Google Scholar] [CrossRef]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K.; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.S.; Nagel, K.; Duckworth, J.; Bissessar, H.; Fischer, M.A.; Portwine, C.; Tozer, R.; Barr, R.D. Effectiveness of sperm banking in adolescents and young adults with cancer: A regional experience. Cancer 2007, 110, 1125–1129. [Google Scholar] [CrossRef]
- Tal, R.; Botchan, A.; Hauser, R.; Yogev, L.; Paz, G.; Yavetz, H. Follow-up of sperm concentration and motility in patients with lymphoma. Hum. Reprod. 2000, 15, 1985–1988. [Google Scholar] [CrossRef] [PubMed]
- Giwercman, A.; Petersen, P.M. Cancer and male infertility. Baillieres Best. Pract. Res. Clin. Endocrinol. Metab. 2000, 14, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Trombetta, C.; Bucci, S.; Benvenuto, S.; Amodeo, A.; Ocello, G.; Belgrano, E. Semen quality before and after orchiectomy in men with testicular cancer. Arch. Ital. Urol. Androl. 2008, 80, 99–102. [Google Scholar] [PubMed]
- Eisenberg, M.L.; Betts, P.; Herder, D.; Lamb, D.J.; Lipshultz, L.I. Increased risk of cancer among azoospermic men. Fertil. Steril. 2013, 100, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K.; American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- van Casteren, N.J.; Boellaard, W.P.; Romijn, J.C.; Dohle, G.R. Gonadal dysfunction in male cancer patients before cytotoxic treatment. Int. J. Androl. 2010, 33, 73–79. [Google Scholar] [CrossRef]
- Agarwal, A.; Ranganathan, P.; Kattal, N.; Pasqualotto, F.; Hallak, J.; Khayal, S.; Mascha, E. Fertility after cancer: A prospective review of assisted reproductive outcome with banked semen specimens. Fertil. Steril. 2004, 81, 342–348. [Google Scholar] [CrossRef]
- Dunn, H.G.; McBurney, A.K.; Ingram, S.; Hunter, C.M. Maternal cigarette smoking during pregnancy and the child’s subsequent development: I. Physical growth to the age of 6 1/2 years. Can. J. Public. Health 1976, 67, 499–505. [Google Scholar]
- Jacobsen, R.; Bostofte, E.; Engholm, G.; Hansen, J.; Olsen, J.H.; Skakkebaek, N.E.; Moller, H. Risk of testicular cancer in men with abnormal semen characteristics: Cohort study. BMJ 2000, 321, 789–792. [Google Scholar] [CrossRef]
- Meseguer, M.; Molina, N.; Garcia-Velasco, J.A.; Remohi, J.; Pellicer, A.; Garrido, N. Sperm cryopreservation in oncological patients: A 14-year follow-up study. Fertil. Steril. 2006, 85, 640–645. [Google Scholar] [CrossRef]
- Kandil, H.; Agarwal, A.; Saleh, R.; Boitrelle, F.; Arafa, M.; Vogiatzi, P.; Henkel, R.; Zini, A.; Shah, R. Editorial Commentary on Draft of World Health Organization Sixth Edition Laboratory Manual for the Examination and Processing of Human Semen. World J. Mens. Health 2021, 39, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Barratt, C.L.R.; Bjorndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil. Steril. 2021, 115, 54–61. [Google Scholar] [CrossRef]
- Nunez-Corona, D.; Contreras-Sanzon, E.; Puente-Rivera, J.; Arreola, R.; Camacho-Nuez, M.; Cruz Santiago, J.; Estrella-Parra, E.A.; Torres-Romero, J.C.; Lopez-Camarillo, C.; Alvarez-Sanchez, M.E. Epigenetic Factors and ncRNAs in Testicular Cancer. Int. J. Mol. Sci. 2023, 24, 2194. [Google Scholar] [CrossRef] [PubMed]
- Donatti, L.M.; Martello, C.L.; Andrade, G.M.; Oliveira, N.P.; Frantz, N. Advanced Paternal Age Affects the Sperm DNA Fragmentation Index and May Lead to Lower Good-quality Blastocysts. Reprod. Sci. 2023, 30, 2489–2494. [Google Scholar] [CrossRef]
- van Casteren, N.J.; van Santbrink, E.J.; van Inzen, W.; Romijn, J.C.; Dohle, G.R. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil. Steril. 2008, 90, 2245–2250. [Google Scholar] [CrossRef]
- Shankara-Narayana, N.; Di Pierro, I.; Fennell, C.; Ly, L.P.; Bacha, F.; Vrga, L.; Savkovic, S.; Turner, L.; Jayadev, V.; Conway, A.J.; et al. Sperm cryopreservation prior to gonadotoxic treatment: Experience of a single academic centre over 4 decades. Hum. Reprod. 2019, 34, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, B.; Liu, S.; Xian, Y.; Zhao, W.; Zhou, B.; Xiao, X.; Wang, L.; Zhu, X.; Shu, B.; et al. Male cancer patient sperm cryopreservation for fertility preservation: 10-year monocentric experience. Basic. Clin. Androl. 2021, 31, 24. [Google Scholar] [CrossRef] [PubMed]
- Rajpert-De Meyts, E.; Aksglaede, L.; Bandak, M.; Toppari, J.; Jorgensen, N. Testicular Cancer: Pathogenesis, Diagnosis and Management with Focus on Endocrine Aspects. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Cannarella, R.; Gul, M.; Rambhatla, A.; Agarwal, A. Temporal decline of sperm concentration: Role of endocrine disruptors. Endocrine 2023, 79, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Masliukaite, I.; Ntemou, E.; Feijen, E.A.M.; van de Wetering, M.; Meissner, A.; Soufan, A.T.; Repping, S.; Kremer, L.M.C.; Jahnukainen, K.; Goossens, E.; et al. Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: A retrospective study of a male fertility preservation cohort. Hum. Reprod. 2023, 38, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Cosci, I.; Grande, G.; Di Nisio, A.; Rocca, M.S.; Del Fiore, P.; Benna, C.; Mocellin, S.; Ferlin, A. Cutaneous Melanoma and Hormones: Focus on Sex Differences and the Testis. Int. J. Mol. Sci. 2022, 24, 599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar Ghosh, S.; Katiyar, R.; Gemeda, A.E.; Rautela, R.; Bisla, A.; Srivastava, N.; Kumar Bhure, S.; Devi, H.L.; Chandra, V. Supplementation of Mito TEMPO and acetovanillone in semen extender improves freezability of buffalo spermatozoa. Andrology 2022, 10, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Zauli, G.; Rimondi, E.; Gianesini, S.; Brunelli, L.; Menegatti, E.; Zamboni, P.; Secchiero, P. Inhibitory effect of natural anti-inflammatory compounds on cytokines released by chronic venous disease patient-derived endothelial cells. Mediators Inflamm. 2013, 2013, 423407. [Google Scholar] [CrossRef]
- Szabo, A.; Vancsa, S.; Hegyi, P.; Varadi, A.; Forintos, A.; Filipov, T.; Acs, J.; Acs, N.; Szarvas, T.; Nyirady, P.; et al. Lifestyle-, environmental-, and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 5. [Google Scholar] [CrossRef]
- Nouman, H.; Zanbar, L.P. Support or stressor? The community as a predictor of perceptions of infertility. Soc. Work. Health Care 2020, 59, 650–667. [Google Scholar] [CrossRef]
- Thanscheidt, C.L.; Patsch, P.; Rosner, S.; Germeyer, A.; Krause, M.; Kentenich, H.; Siercks, I.; Haberlin, F.; Ehrbar, V.; Tschudin, S.; et al. Psychological Aspects of Infertility-Results from an Actor-Partner Interdependence Analysis. Geburtshilfe Frauenheilkd. 2023, 83, 843–849. [Google Scholar] [CrossRef]
- Sharma, P.; Kaushal, N.; Saleth, L.R.; Ghavami, S.; Dhingra, S.; Kaur, P. Oxidative stress-induced apoptosis and autophagy: Balancing the contrary forces in spermatogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166742. [Google Scholar] [CrossRef]
- Tisato, V.; Zuliani, G.; Vigliano, M.; Longo, G.; Franchini, E.; Secchiero, P.; Zauli, G.; Paraboschi, E.M.; Vikram Singh, A.; Serino, M.L.; et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PLoS ONE 2018, 13, e0193867. [Google Scholar] [CrossRef]
- Castiglione, A.; Ciorba, A.; Aimoni, C.; Orioli, E.; Zeri, G.; Vigliano, M.; Gemmati, D. Sudden sensorineural hearing loss and polymorphisms in iron homeostasis genes: New insights from a case-control study. Biomed. Res. Int. 2015, 2015, 834736. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Zeri, G.; Orioli, E.; De Gaetano, F.E.; Salvi, F.; Bartolomei, I.; D’Alfonso, S.; Dall’osso, C.; Leone, M.A.; Singh, A.V.; et al. Polymorphisms in the genes coding for iron binding and transporting proteins are associated with disability, severity, and early progression in multiple sclerosis. BMC Med. Genet. 2012, 13, 70. [Google Scholar] [CrossRef]
- Zamboni, P.; Gemmati, D. Clinical implications of gene polymorphisms in venous leg ulcer: A model in tissue injury and reparative process. Thromb. Haemost. 2007, 98, 131–137. [Google Scholar]
- Parmeggiani, F.; Costagliola, C.; Gemmati, D.; D’Angelo, S.; Perri, P.; Scapoli, G.L.; Catozzi, L.; Federici, F.; Sebastiani, A.; Incorvaia, C. Predictive role of coagulation-balance gene polymorphisms in the efficacy of photodynamic therapy with verteporfin for classic choroidal neovascularization secondary to age-related macular degeneration. Pharmacogenet Genom. 2007, 17, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, P.S.; Kaneko-Tarui, T.; Zhang, L.; Teixeira, J.M. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum. Mol. Genet. 2012, 21, 4394–4405. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Q.; Wei, B.H.; Hao, S.L.; Yang, W.X. The PI3K/AKT signaling pathway: How does it regulate development of Sertoli cells and spermatogenic cells? Histol. Histopathol. 2022, 37, 621–636. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Song, W.; Huang, T.; Wang, Y.; Chen, Z.; Chen, F.; Liu, Y.; Wang, X.; Jiang, Y.; et al. Review of the Role of Ferroptosis in Testicular Function. Nutrients 2022, 14, 5268. [Google Scholar] [CrossRef]
- Gemmati, D.; Tisato, V. Chapter 24-Genomic and epigenomic signature at the branch-point among genome, phenome, and sexome in health and disease: A multiomics approach. In Principles of Gender-Specific Medicine, 4th ed.; Legato, M.J., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 393–408. [Google Scholar]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Care, A.; Bellini, T. “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef]
- Hardy, J.; Pollock, N.; Gingrich, T.; Sweet, P.; Ramesh, A.; Kuong, J.; Basar, A.; Jiang, H.; Hwang, K.; Vukina, J.; et al. Genomic testing for copy number and single nucleotide variants in spermatogenic failure. J. Assist. Reprod. Genet. 2022, 39, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Beaud, H.; Tremblay, A.R.; Chan, P.T.K.; Delbes, G. Sperm DNA Damage in Cancer Patients. Adv. Exp. Med. Biol. 2019, 1166, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.B.R.; Bertolla, R.P.; Intasqui, P.; Antoniassi, M.P.; Tibaldi, D.S.; Belardin, L.B.; Spaine, D.M. Effect of orchiectomy on sperm functional aspects and semen oxidative stress in men with testicular tumours. Andrologia 2019, 51, e13205. [Google Scholar] [CrossRef] [PubMed]
- Lackamp, N.; Wilkemeyer, I.; Jelas, I.; Keller, U.; Bullinger, L.; Stintzing, S.; le Coutre, P. Survey of Long-Term Experiences of Sperm Cryopreservation in Oncological and Non-Oncological Patients: Usage and Reproductive Outcomes of a Large Monocentric Cohort. Front. Oncol. 2021, 11, 772809. [Google Scholar] [CrossRef]
- Tremblay, A.; Beaud, H.; Delbès, G. Transgenerational impact of chemotherapy: Would the father exposure impact the health of future progeny? Gynecol. Obstet. Fertil. Senol. 2017, 45, 609–618. [Google Scholar] [CrossRef]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy During Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef]




| Type of Cancer | ≤15 × 106/mL | >15 × 106/mL | p-Value * |
|---|---|---|---|
| a Testicular cancer (n = 135) | 70.37% (95) | 29.63% (40) | 0.003 (a vs. b) |
| b Hematol. cancers (n = 76) | 50.0% (38) | 50.0% (38) | 0.06 (a vs. c) |
| c Other cancers (n = 43) | 55.8% (24) | 44.2% (19) | 0.0028 (a vs. b + c) |
| Whole Cohort | Hematol. Cancers | Other Cancers | Testicular Cancer | p-Value | WHO 2021 | |
|---|---|---|---|---|---|---|
| Age, mean ± SD | 30.2 ± 7.6 | 28.4 ± 8.5 | 31.9 ± 9.2 | 30.7 ± 6.1 | 0.02 a n.s. b | - - |
| Ejaculate volume, mL | 2.2 ± 1.3 | 2.03 ± 1.2 | 2.48 ± 1.5 | 2.2 ± 1.3 | n.s. a n.s. b | 1.4 (1.3–1.5) |
| Number/mL, ×106 | 21.2 ± 28.7 | 26.9 ± 28.3 | 29.6 ± 39.9 | 15.3 ± 23.0 | 0.0014 a 0.004 b | 39 (35–40) |
| Total motility, % | 18.2 ± 17.8 | 21.7 ± 18.6 | 19.1 ± 20 | 16.0 ± 16.3 | 0.02 a 0.07 b | 42 (40–43) |
| Progressive motility, % | 6.6 ± 8.9 | 8.02 ± 9.4 | 7.1 ± 10.6 | 5.7 ± 7.9 | 0.04 a 0.05 b | 30 (29–31) |
| Viability, % | 62.3 ± 18.7 | 67.1 ± 16.3 | 59.4 ± 18.4 | 60.3 ± 19.9 | 0.01 a n.s. b | 54 (50–56) |
| Morphology, % | 7.73 ± 8.4 | 8.75 ± 8.7 | 9.56 ± 9.8 | 6.59 ± 7.5 | 0.05 a 0.03 b | 4 (3.9–4) |
| Pre-Cryopreservation | ||||
|---|---|---|---|---|
| Post-Cryopreservation | Viability (%) | Progressive Motility (%) | ||
| Whole cohort | Viability, % | R2 = 0.35; p < 0.001 | R2 = 0.42; p < 0.001 | |
| Progressive motility, % | R2 = 0.35; p < 0.001 | R2 = 0.45; p < 0.001 | ||
| Hematological cancers (HC) | Viability, % | R2 = 0.12; p = n.s. | R2 = 0.16; p = n.s. | |
| Progressive motility, % | R2 = 0.18; p = n.s. | R2 = 0.13; p = n.s. | ||
| Other cancers (OC) | Viability, % | R2 = 0.09; p = n.s | R2 = 0.06; p = n.s | |
| Progressive motility, % | R2 = 0.08; p = n.s | R2 = 0.07; p = n.s | ||
| Testis cancer (TC) | Viability, % | R2 = 0.2; p < 0.05 | R2 = 0.18; p < 0.05 | |
| Progressive motility, % | R2 = 0.16; p < 0.05 | R2 = 0.2; p < 0.05 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peluso, G.; Tisato, V.; Singh, A.V.; Gemmati, D.; Scarpellini, F. Semen Cryopreservation to Expand Male Fertility in Cancer Patients: Intracase Evaluation of Semen Quality. J. Pers. Med. 2023, 13, 1654. https://doi.org/10.3390/jpm13121654
Peluso G, Tisato V, Singh AV, Gemmati D, Scarpellini F. Semen Cryopreservation to Expand Male Fertility in Cancer Patients: Intracase Evaluation of Semen Quality. Journal of Personalized Medicine. 2023; 13(12):1654. https://doi.org/10.3390/jpm13121654
Chicago/Turabian StylePeluso, Giuseppina, Veronica Tisato, Ajay Vikram Singh, Donato Gemmati, and Fabio Scarpellini. 2023. "Semen Cryopreservation to Expand Male Fertility in Cancer Patients: Intracase Evaluation of Semen Quality" Journal of Personalized Medicine 13, no. 12: 1654. https://doi.org/10.3390/jpm13121654