Exploring the Safety of Pllans-II and Antitumoral Potential of Its Recombinant Isoform in Cervical Cancer Therapy
Abstract
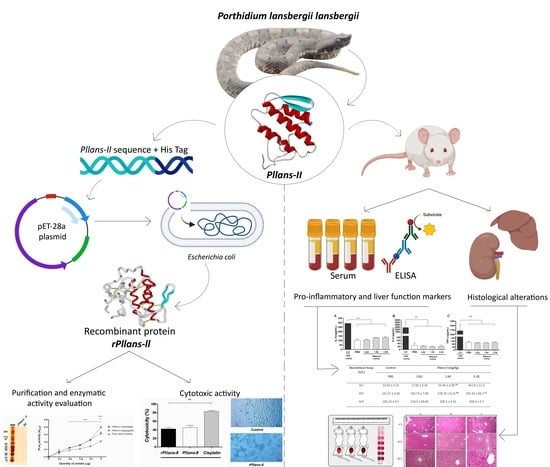
:1. Introduction
2. Materials and Methods
2.1. Snake Venom Collection and Protein Purification
2.2. Animals
2.3. Analysis of Pro-Inflammatory Markers and Liver Damage
2.4. Histological Alterations Analysis
2.5. Local Hemorrhage Activity
2.6. Production, Purification of Recombinant rPllans-II, and Evaluation of Its Activity
2.7. Determination of Phospholipase Activity
2.8. Cell Cultures
2.9. Cytotoxicity
2.10. Statistical Analysis
3. Results
3.1. Pllans-II Inoculation on Murine Biomodels
3.1.1. Pro-Inflammatory Markers and Liver Damage Analysis
3.1.2. Histological Analysis
3.1.3. Local Hemorrhage
3.2. rPllans-II Production and Evaluation of Its Anticancer Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naughton, M.J.; Weaver, K.E. Physical and mental health among cancer survivors: Considerations for long-term care and quality of life. N. C. Med. J. 2014, 75, 283–286. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C. Image Guided Brachytherapy in Locally Advanced Cervical Cancer: Improved Pelvic Control and Survival in RetroEMBRACE, a Multicenter Cohort Study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Montealegre-Sánchez, L.; Gimenes, S.N.C.; Lopes, D.S.; Teixeira, S.C.; Solano-Redondo, L.; de Melo Rodrigues, V.; Jiménez-Charris, E. Antitumoral Potential of Lansbermin-I, a Novel Disintegrin from Porthidium lansbergii lansbergii Venom on Breast Cancer Cells. Curr. Top. Med. Chem. 2019, 19, 2069–2078. [Google Scholar] [CrossRef]
- Montoya-Gómez, A.; Montealegre-Sánchez, L.; García-Perdomo, H.A.; Jiménez-Charris, E. Cervical Cancer and Potential Pharmacological Treatment with Snake Venoms. Mol. Biol. Rep. 2020, 47, 4709–4721. [Google Scholar] [CrossRef]
- De Oliveira Guimarães, D.; Lopes, D.S.; Azevedo, F.V.P.V.; Gimenes, S.N.C.; Silva, M.A.; Ache, D.C.; Gomes, M.S.R.; Vecchi, L.; Goulart, L.R.; Yoneyama, K.A.G. In Vitro Antitumor and Antiangiogenic Effects of Bothropoidin, a Metalloproteinase from Bothrops Pauloensis Snake Venom. Int. J. Biol. Macromol. 2017, 97, 770–777. [Google Scholar] [CrossRef]
- Silva, M.A.; Lopes, D.S.; Teixeira, S.C.; Gimenes, S.N.C.; Azevedo, F.V.P.V.; Polloni, L.; Borges, B.C.; da Silva, M.S.; Barbosa, M.J.; de Oliveira Junior, R.J. Genotoxic Effects of BnSP-6, a Lys-49 Phospholipase A2 (PLA2) Homologue from Bothrops Pauloensis Snake Venom, on MDA-MB-231 Breast Cancer Cells. Int. J. Biol. Macromol. 2018, 118, 311–319. [Google Scholar] [CrossRef]
- De Vasconcelos Azevedo, F.V.P.; Zóia, M.A.P.; Lopes, D.S.; Gimenes, S.N.; Vecchi, L.; Alves, P.T.; Rodrigues, R.S.; Silva, A.C.A.; Yoneyama, K.A.G.; Goulart, L.R. Antitumor and Antimetastatic Effects of PLA2-BthTX-II from Bothrops Jararacussu Venom on Human Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 135, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Jiménez–Charris, E.; Lopes, D.S.; Gimenes, S.N.C.; Teixeira, S.C.; Montealegre–Sánchez, L.; Solano–Redondo, L.; Fierro–Pérez, L.; de Melo Rodrigues Ávila, V. Antitumor Potential of Pllans–II, an Acidic Asp49–PLA2 from Porthidium Lansbergii Lansbergii Snake Venom on Human Cervical Carcinoma HeLa Cells. Int. J. Biol. Macromol. 2019, 122, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Gómez, A.; Franco, N.R.; Montealegre-Sanchez, L.I.; Solano-Redondo, L.M.; Castillo, A.; Mosquera-Escudero, M.; Jiménez-Charris, E. Pllans–II Induces Cell Death in Cervical Cancer Squamous Epithelial Cells via Unfolded Protein Accumulation and Endoplasmic Reticulum Stress. Molecules 2022, 27, 6491. [Google Scholar] [CrossRef]
- Jiménez-Charris, E.; Montealegre-Sanchez, L.; Solano-Redondo, L.; Mora-Obando, D.; Camacho, E.; Castro-Herrera, F.; Fierro-Pérez, L.; Lomonte, B. Proteomic and Functional Analyses of the Venom of Porthidium Lansbergii Lansbergii (Lansberg’s Hognose Viper) from the Atlantic Department of Colombia. J. Proteom. 2015, 114, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Charris, E.; Montealegre-Sánchez, L.; Solano-Redondo, L.; Castro-Herrera, F.; Fierro-Pérez, L.; Lomonte, B. Divergent Functional Profiles of Acidic and Basic Phospholipases A2 in the Venom of the Snake Porthidium Lansbergii Lansbergii. Toxicon 2016, 119, 289–298. [Google Scholar] [CrossRef]
- Roth, A.; Singer, T. The Application of 3D Cell Models to Support Drug Safety Assessment: Opportunities & Challenges. Adv. Drug Deliv. Rev. 2014, 69, 179–189. [Google Scholar] [CrossRef]
- Astashkina, A.; Mann, B.; Grainger, D.W. A Critical Evaluation of in Vitro Cell Culture Models for High-Throughput Drug Screening and Toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef]
- Hou, S.; Hsia, C.; Velusamy, M.; Jayakumar, T.; Hsia, C.; Chang, C.; Lin, K.; Lu, Y. Ruthenium Complex, TQ-5, Protects against LPS-induced Macrophage Inflammation and Acute Liver Injury in Mice via Downregulating NF-κB Pathways. Int. J. Mol. Med. 2019, 44, 335–345. [Google Scholar] [CrossRef]
- Salazar, E.; Salazar, A.M.; Taylor, P.; Ibarra, C.; Rodríguez-Acosta, A.; Sánchez, E.; Pérez, K.; Brito, B.; Guerrero, B. Pro-Inflammatory Response and Hemostatic Disorder Induced by Venom of the Coral Snake Micrurus Tener Tener IN C57BL/6 Mice. Toxicon 2018, 150, 212–219. [Google Scholar] [CrossRef]
- Khalil, A.M.; Wahsha, M.A.; Khadra, K.M.A.; Khalaf, M.A.; Al-Najjar, T.H. Biochemical and Histopathological Effects of the Stonefish (Synanceia Verrucosa) Venom in Rats. Toxicon 2018, 142, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Schoell, A.R.; Heyde, B.R.; Weir, D.E.; Chiang, P.-C.; Hu, Y.; Tung, D.K. Euthanasia Method for Mice in Rapid Time-Course Pulmonary Pharmacokinetic Studies. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 506–511. [Google Scholar] [PubMed]
- Jenkins, T.P.; Sánchez, A.; Segura, Á.; Vargas, M.; Herrera, M.; Stewart, T.K.; León, G.; Gutiérrez, J.M. An Improved Technique for the Assessment of Venom-Induced Haemorrhage in a Murine Model. Toxicon 2017, 139, 87–93. [Google Scholar] [CrossRef] [PubMed]
- De arco-Rodríguez, B.; Montealegre-Sánchez, L.; Solano-Redondo, L.; Castro-Herrera, F.; Ortega, J.G.; Castillo, A.; Vargas-Zapata, C.; Jiménez-Charris, E. Phylogeny and Toxicological Assessments of Two Porthidium lansbergii lansbergii Morphotypes from the Caribbean Region of Colombia. Toxicon 2019, 166, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Di Veroli, G.Y.; Fornari, C.; Goldlust, I.; Mills, G.; Koh, S.B.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. An Automated Fitting Procedure and Software for Dose-Response Curves with Multiphasic Features. Sci. Rep. 2015, 5, 14701. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.C.; Zuliani, J.P.; et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Chisari, A.; Spinedi, E.; Voirol, M.J.; Giovambattista, A.; Gaillard, R.C. A phospholipase A2-related snake venom (from Crotalus durissus terrificus) stimulates neuroendocrine and immune functions: Determination of different sites of action. Endocrinology 1998, 139, 617–625. [Google Scholar] [CrossRef]
- Cedro, R.C.; Menaldo, D.L.; Costa, T.R.; Zoccal, K.F.; Sartim, M.A.; Santos-Filho, N.A.; Faccioli, L.H.; Sampaio, S.V. Cytotoxic and inflammatory potential of a phospholipase A2 from Bothrops jararaca snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Fontana, B.C.; Soares, A.M.; Zuliani, J.P.; Gonçalves, G.M. Role of Toll-like receptors in local effects in a model of experimental envenoming induced by Bothrops jararacussu snake venom and by two phospholipases A2. Toxicon 2022, 214, 145–154. [Google Scholar] [CrossRef]
- Marinho, A.D.; de Moraes Silveira, J.A.; Chaves Filho, A.J.M.; Jorge, A.R.C.; Júnior, F.A.N.; Pereira, V.B.M.; de Aquino, P.E.A.; Souza Pereira, C.A.; Evangelista, J.S.A.M.; Macedo, D.S.; et al. Bothrops pauloensis snake venom-derived Asp-49 and Lys-49 phospholipases A2 mediates acute kidney injury by oxidative stress and release of inflammatory cytokines. Toxicon 2021, 190, 31–38. [Google Scholar] [CrossRef]
- Zuliani, J.P.; Fernandes, C.M.; Zamuner, S.R.; Gutiérrez, J.M.; Teixeira, C.F.P. Inflammatory Events Induced by Lys-49 and Asp-49 Phospholipases A2 Isolated from Bothrops Asper Snake Venom: Role of Catalytic Activity. Toxicon 2005, 45, 335–346. [Google Scholar] [CrossRef]
- Lomonte, B.; Tarkowski, A.; Hanson, L.Å. Host Response to Bothrops Asper Snake Venom: Analysis of Edema Formation, Inflammatory Cells, and Cytokine Release in a Mouse Model. Inflammation 1993, 17, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-F.; Li, T.; Wei, X.-L.; Sun, Q.-Y.; Yang, F.-M.; Chen, Q.-Y.; Wang, W.-Y.; Xiong, Y.-L.; He, S.-H. Purification, Characterization and Cytokine Release Function of a Novel Arg-49 Phospholipase A2 from the Venom of Protobothrops Mucrosquamatus. Biochimie 2006, 88, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.R.; Carazo, L.P.; Klímová, K. Interpretación Diagnóstica y Pronóstica de Las Pruebas de Función Hepática. Med. Form. Médica Contin. Acreditado 2012, 11, 733–739. [Google Scholar] [CrossRef]
- Busto Bea, V.; Herrero Quirós, C. Pruebas de Función Hepática: B, AST, ALT, FA y GGT. Rev. Española Enfermedades Dig. 2015, 107, 648. [Google Scholar]
- Romero-García, J.G.; Mayon Flores, B.A. Evaluación de La Química Hepática Alterada. REMUS Rev. Estud. Med. Univ. Son. 2022, 7, 43–46. [Google Scholar] [CrossRef]
- Bordon, K.D.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Pino Anjolette, F.A.; Almeida Cordeiro, F.; Adriano Wiezel, G.; Cardoso, I.A.; et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.; Lomonte, B. Phospholipase A2 Myotoxins from Bothrops Snake Venoms. Toxicon 1995, 33, 1405–1424. [Google Scholar] [CrossRef]
- Howes, J.-M.; Theakston, R.D.G.; Laing, G.D. Neutralization of the Haemorrhagic Activities of Viperine Snake Venoms and Venom Metalloproteinases Using Synthetic Peptide Inhibitors and Chelators. Toxicon 2007, 49, 734–739. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Maity, C.R. The Composition of Naja Naja Venom Samples from Three Districts of West Bengal, India. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 621–627. [Google Scholar] [CrossRef]
- Teixeira, C.F.P.; Landucci, E.C.T.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory Effects of Snake Venom Myotoxic Phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B. Lys49 Myotoxins, Secreted Phospholipase A2-like Proteins of Viperid Venoms: A Comprehensive Review. Toxicon 2023, 224, 107024. [Google Scholar] [CrossRef] [PubMed]
- Fuly, A.L.; Calil-Elias, S.; Martinez, A.M.B.; Melo, P.A.; Guimarães, J.A. Myotoxicity Induced by an Acidic Asp-49 Phospholipase A2 Isolated from Lachesis Muta Snake Venom: Comparison with Lysophosphatidylcholine. Int. J. Biochem. Cell Biol. 2003, 35, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Jacob-Ferreira, A.L.; Bernardes, C.P.; Cintra, A.C.O.; Sampaio, S.V. Purification Procedure for the Isolation of a PI Metalloprotease and an Acidic Phospholipase A2 From Bothrops Atrox Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.; Gasparini, S.; Drevet, P.; Ducancel, F.; Bouet, F.; Boulain, J.; Harris, J.B.; Menez, A. Production of Recombinant Notechis 11′ 2L, an Enzymatically Active Mutant of a Phospholipase A2 from Notechis scutatus scutatus Venom, as Directly Generated by Cleavage of a Fusion Protein Produced in Escherichia Coli. Eur. J. Biochem. 1993, 212, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.D.; Iemma, M.R.C.; Bondioli, A.C.V.; Souza, D.H.F.; Ferreira, L.L.; Amaral, A.C.; Salvini, T.F.; Selistre-de-Araujo, H.S. Expression of an Active Recombinant Lysine 49 Phospholipase A2 Myotoxin as a Fusion Protein in Bacteria. Toxicon 2001, 39, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Takemori, D.; Yoshino, K.; Eba, C.; Nakano, H.; Iwasaki, Y. Extracellular Production of Phospholipase A2 from Streptomyces Violaceoruber by Recombinant Escherichia Coli. Protein Expr. Purif. 2012, 81, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Cho, A.; Hwang, Y.; Park, J.-B.; Kim, S.-K. Engineering of a Microbial Cell Factory for the Extracellular Production of Catalytically Active Phospholipase A2 of Streptomyces Violaceoruber. J. Microbiol. Biotechnol. 2020, 30, 1244. [Google Scholar] [CrossRef]
- Russo, R.R.; dos Santos Júnior, N.N.; Cintra, A.C.O.; Figueiredo, L.T.M.; Sampaio, S.V.; Aquino, V.H. Expression, Purification and Virucidal Activity of Two Recombinant Isoforms of Phospholipase A2 from Crotalus durissus terrificus Venom. Arch. Virol. 2019, 164, 1159–1171. [Google Scholar] [CrossRef]
- Landeta, C.; Boyd, D.; Beckwith, J. Disulfide Bond Formation in Prokaryotes. Nat. Microbiol. 2018, 3, 270–280. [Google Scholar] [CrossRef]
- Karyolaimos, A.; Dolata, K.M.; Antelo-Varela, M.; Mestre Borras, A.; Elfageih, R.; Sievers, S.; Becher, D.; Riedel, K.; de Gier, J.-W. Escherichia Coli Can Adapt Its Protein Translocation Machinery for Enhanced Periplasmic Recombinant Protein Production. Front. Bioeng. Biotechnol. 2020, 7, 465. [Google Scholar] [CrossRef]
- Costa, T.R.; Menaldo, D.L.; Oliveira, C.Z.; Santos-Filho, N.A.; Teixeira, S.S.; Nomizo, A.; Fuly, A.L.; Monteiro, M.C.; de Souza, B.M.; Palma, M.S. Myotoxic Phospholipases A2 Isolated from Bothrops Brazili Snake Venom and Synthetic Peptides Derived from Their C-Terminal Region: Cytotoxic Effect on Microorganism and Tumor Cells. Peptides 2008, 29, 1645–1656. [Google Scholar] [CrossRef]
- Gebrim, L.C.; Marcussi, S.; Menaldo, D.L.; de Menezes, C.S.R.; Nomizo, A.; Hamaguchi, A.; Silveira-Lacerda, E.P.; Homsi-Brandeburgo, M.I.; Sampaio, S.V.; Soares, A.M. Antitumor Effects of Snake Venom Chemically Modified Lys49 Phospholipase A2-like BthTX-I and a Synthetic Peptide Derived from Its C-Terminal Region. Biologicals 2009, 37, 222–229. [Google Scholar] [CrossRef]
- Lomonte, B.; Angulo, Y.; Moreno, E. Synthetic Peptides Derived from the C-Terminal Region of Lys49 Phospholipase A2 Homologues from Viperidae Snake Venoms: Biomimetic Activities and Potential Applications. Curr. Pharm. Des. 2010, 16, 3224–3230. [Google Scholar] [CrossRef]





| Biochemical Assay (U/L) | Control | Pllans-II (mg/kg) | ||
|---|---|---|---|---|
| PBS | 0.82 | 1.64 | 3.28 | |
| ALT | 33.55 ± 4.15 | 27.65 ± 3.45 | 54.45 ± 4.05 (a) | 44.55 ± 11.5 |
| AST | 131.37 ± 0.35 | 102.73 ± 7.85 | 278.75 ± 9.15 (b) | 335.53 ± 66.7 (c) |
| ALP | 205.25 ± 4.5 | 216.5 ± 49.65 | 205.5 ± 9.33 | 204.5 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevilla-Sánchez, M.J.; Montoya-Gómez, A.; Osorno-Valencia, D.; Montealegre-Sánchez, L.; Mosquera-Escudero, M.; Jiménez-Charris, E. Exploring the Safety of Pllans-II and Antitumoral Potential of Its Recombinant Isoform in Cervical Cancer Therapy. Cells 2023, 12, 2812. https://doi.org/10.3390/cells12242812
Sevilla-Sánchez MJ, Montoya-Gómez A, Osorno-Valencia D, Montealegre-Sánchez L, Mosquera-Escudero M, Jiménez-Charris E. Exploring the Safety of Pllans-II and Antitumoral Potential of Its Recombinant Isoform in Cervical Cancer Therapy. Cells. 2023; 12(24):2812. https://doi.org/10.3390/cells12242812
Chicago/Turabian StyleSevilla-Sánchez, María José, Alejandro Montoya-Gómez, Daniel Osorno-Valencia, Leonel Montealegre-Sánchez, Mildrey Mosquera-Escudero, and Eliécer Jiménez-Charris. 2023. "Exploring the Safety of Pllans-II and Antitumoral Potential of Its Recombinant Isoform in Cervical Cancer Therapy" Cells 12, no. 24: 2812. https://doi.org/10.3390/cells12242812