Table Olive Manufacturing Wastewater Treatment Using the Peroxymonosulfate/Fe(III) System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Coagulation–Flocculation Stage
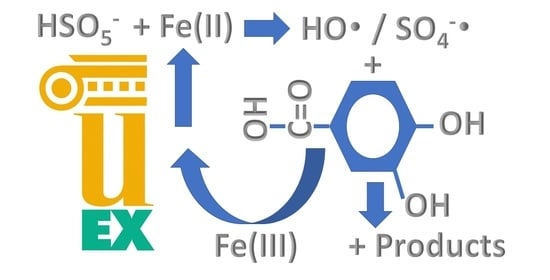
2.2. Application of the Fe(III)/PMS System
2.2.1. Influence of Initial PMS Concentration
2.2.2. Influence of Initial Fe(III) Concentration
2.2.3. pH Influence
- -
- -
- A pH level of above 3.0 may lead to Fe(III) precipitation, thus inhibiting its catalytic role in PMS decomposition.
- -
- For pH below 5.0, radicals can be scavenged by protons [24].
- -
- The efficacy of the PMS/Fe(III)/Fe(II) system at low pH can be reduced due to the formation of iron aquocomplexes (the reduction of the available free iron) [24].
- -
- The reactivity of target pollutants can significantly differ depending on the major structure present (protonated, neutral, or ionic forms). This is especially relevant in phenol-type substances.
- -
- Extremely acidic pH may prompt proton bonding to the O−O bond of PMS, diminishing and inhibiting the decomposition with Fe(II) [25].
3. Materials and Methods
3.1. Wastewater Characterization
3.2. Analytical Procedure
3.3. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A Review of the Recent Advances on the Treatment of Industrial Wastewaters by Sulfate Radical-Based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Berruti, I.; Nahim-Granados, S.; Abeledo-Lameiro, M.J.; Oller, I.; Polo-López, M.I. Peroxymonosulfate/Solar Process for Urban Wastewater Purification at a Pilot Plant Scale: A Techno-Economic Assessment. Sci. Total Environ. 2023, 881, 163407. [Google Scholar] [CrossRef]
- Yin, L.; Wei, J.; Qi, Y.; Tu, Z.; Qu, R.; Yan, C.; Wang, Z.; Zhu, F. Degradation of Pentachlorophenol in Peroxymonosulfate/Heat System: Kinetics, Mechanism, and Theoretical Calculations. Chem. Eng. J. 2022, 434, 134736. [Google Scholar] [CrossRef]
- Al Hakim, S.; Baalbaki, A.; Tantawi, O.; Ghauch, A. Chemically and Thermally Activated Persulfate for Theophylline Degradation and Application to Pharmaceutical Factory Effluent. RSC Adv. 2019, 9, 33472–33485. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Garcia-Cañibano, C.; Sarro, M.; Encinas, Á.; Medana, C.; Fabbri, D.; Calza, P.; Marugán, J. Evaluation of Transformation Products from Chemical Oxidation of Micropollutants in Wastewater by Photoassisted Generation of Sulfate Radicals. Chemosphere 2019, 226, 509–519. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Manoli, K.; Ji, Y.; Yu, X.; Feng, M. Degradation of Methotrexate by Unactivated and Solar-Activated Peroxymonosulfate in Water: Moiety-Specific Reaction Kinetics and Transformation Product-Associated Risks. Water Res. 2023, 246, 120741. [Google Scholar] [CrossRef]
- Hou, K.; Pi, Z.; Chen, F.; He, L.; Yao, F.; Chen, S.; Li, X.; Dong, H.; Yang, Q. Sulfide Enhances the Fe(II)/Fe(III) Cycle in Fe(III)-Peroxymonosulfate System for Rapid Removal of Organic Contaminants: Treatment Efficiency, Kinetics and Mechanism. J. Hazard. Mater. 2022, 435, 128970. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The Interactions of Polyphenols with Fe and Their Application in Fenton/Fenton-like Reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Zhang, Z.; Sun, L.; Peng, Y. Enhancement of Ciprofloxacin Degradation in the Fe(II)/Peroxymonosulfate System by Protocatechuic Acid over a Wide Initial pH Range. Chem. Eng. J. 2019, 372, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Z.; Wang, L.; Jin, P.; Yang, S. Gallic Acid Enhanced Bisphenol A Degradation through Fe3+/Peroxymonosulfate Process. Water Supply 2022, 22, 4852–4863. [Google Scholar] [CrossRef]
- Rincón-Llorente, B.; la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Borja-Padilla, R. Table Olive Wastewater: Problem, Treatments and Future Strategy. A Review. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef]
- Achak, M.; Elayadi, F.; Boumya, W. Chemical Coagulation/Flocculation Processes for Removal of Phenolic Compounds from Olive Mill Wastewater: A Comprehensive Review. Am. J. Appl. Sci. 2019, 16, 59–91. [Google Scholar] [CrossRef]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.J.; Ratnayaka, D.D. Chapter 8—Storage, Clarification and Chemical Treatment. In Twort’s Water Supply; Brandt, M.J., Johnson, K.M., Elphinston, A.J., Ratnayaka, D.D., Seventh, E., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 323–366. ISBN 978-0-08-100025-0. [Google Scholar]
- Aldana, J.C.; Acero, J.L.; Álvarez, P.M. Membrane Filtration, Activated Sludge and Solar Photocatalytic Technologies for the Effective Treatment of Table Olive Processing Wastewater. J. Environ. Chem. Eng. 2021, 9, 105743. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Iborra-Clar, A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Fermentation Brines from Spanish Style Green Table Olives Processing: Treatment Alternatives before Recycling or Recovery Operations. J. Chem. Technol. Biotechnol. 2016, 91, 131–137. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.J.; Gimeno, O.; Alvarez, P. Treatment of Brines by Combined Fenton’s Reagent–Aerobic Biodegradation: II. Process Modelling. J. Hazard. Mater. 2003, 96, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Duesterberg, C.K.; Waite, T.D. Kinetic Modeling of the Oxidation of P-Hydroxybenzoic Acid by Fenton’s Reagent: Implications of the Role of Quinones in the Redox Cycling of Iron. Environ. Sci. Technol. 2007, 41, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.Y.; Abdelraheem, W.H.; He, X.; Kestioglu, K.; Dionysiou, D.D. Photochemical Treatment of Tyrosol, a Model Phenolic Compound Present in Olive Mill Wastewater, by Hydroxyl and Sulfate Radical-Based Advanced Oxidation Processes (AOPs). J. Hazard. Mater. 2019, 367, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Vaz, T.; Domingues, E.; Gomes, J.; Martins, R.C. Evaluation of the Activation Procedure on Oxone Efficiency for Synthetic Olive Mill Wastewater Treatment. Catalysts 2022, 12, 291. [Google Scholar] [CrossRef]
- Can-Güven, E.; Bayrak, Ş.; Dokuyucu, B.; Yazici Guvenc, S.; Varank, G. Hybrid Treatment of Olive Mill Wastewater by Iron-Catalyzed Percarbonate and Peroxymonosulfate Oxidation Followed by Electrooxidation. J. Water Process Eng. 2023, 54, 104018. [Google Scholar] [CrossRef]
- Rivas, F.J. Monopersulfate in Water Treatment: Kinetics. J. Hazard. Mater. 2022, 430, 128383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chu, W. Degradation of 2,4,5-Trichlorophenoxyacetic Acid by a Novel Electro-Fe(II)/Oxone Process Using Iron Sheet as the Sacrificial Anode. Water Res. 2011, 45, 3883–3889. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate Radical-Based Ferrous–Peroxymonosulfate Oxidative System for PCBs Degradation in Aqueous and Sediment Systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, D.; Chu, W.; Li, M.; Lu, X. Nanoscaled Magnetic CuFe2O4 as an Activator of Peroxymonosulfate for the Degradation of Antibiotics Norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Yu, J.; Cui, J.; Zhang, C. ChemInform Abstract: A Simple and Effective Method for α-Hydroxylation of β-Dicarbonyl Compounds Using Oxone as an Oxidant Without a Catalyst. Eur. J. Org. Chem. 2010, 2010, 7020–7026. [Google Scholar] [CrossRef]
- Priewisch, B.; Rück-Braun, K. Efficient Preparation of Nitrosoarenes for the Synthesis of Azobenzenes. J. Org. Chem. 2005, 70, 2350–2352. [Google Scholar] [CrossRef]
- Fields, J.D.; Kropp, P.J. Surface-Mediated Reactions. 9. Selective Oxidation of Primary and Secondary Amines to Hydroxylamines1. J. Org. Chem. 2000, 65, 5937–5941. [Google Scholar] [CrossRef]
- Yan, J.; Travis, B.R.; Borhan, B. Direct Oxidative Cleavage of Alpha- and Beta-Dicarbonyls and Alpha-Hydroxyketones to Diesters with KHSO5. J. Org. Chem. 2004, 69, 9299–9302. [Google Scholar] [CrossRef]
- Rivas, F.J.; Gimeno, O.; Borallho, T. Aqueous Pharmaceutical Compounds Removal by Potassium Monopersulfate. Uncatalyzed and Catalyzed Semicontinuous Experiments. Chem. Eng. J. 2012, 192, 326–333. [Google Scholar] [CrossRef]
- Fortnum, D.H.; Battaglia, C.J.; Cohen, S.R.; Edwards, J.O. The Kinetics of the Oxidation of Halide Ions by Monosubstituted Peroxides. J. Am. Chem. Soc. 1960, 82, 778–782. [Google Scholar] [CrossRef]
- Rivas, F.J.; Solís, R.R. Chloride Promoted Oxidation of Tritosulfuron by Peroxymonosulfate. Chem. Eng. J. 2018, 349, 728–736. [Google Scholar] [CrossRef]
- Moore, W.A.; Kroner, R.C.; Ruchhoft, C.C. Dichromate Reflux Method for Determination of Oxygen Consumed. Anal. Chem. 1949, 21, 953–957. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Eaton, A.D.; Franson, M.A.H.; Association, A.P.H.; Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; Apha, Standard Method for the Examination Fo Water and Waste; American Public Health Association: Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]









| pH | 8.02 ± 0.001 |
| Conductivity, mS/cm | 74.8 ± 0.7 |
| Total organic carbon, ppm | 10,400 ± 150 |
| Inorganic carbon, ppm | 242.5 ± 0.1 |
| Nitrates, ppm | 92 ± 1.53 |
| Polyphenols (ppm gallic acid) | 1081 ± 31 |
| Chemical oxygen demand, ppm | 34,033 ± 1318 |
| Turbidity, NTU units | 187 ± 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, F.J.; Beltrán, F.J.; Gimeno, O. Table Olive Manufacturing Wastewater Treatment Using the Peroxymonosulfate/Fe(III) System. Catalysts 2024, 14, 121. https://doi.org/10.3390/catal14020121
Rivas FJ, Beltrán FJ, Gimeno O. Table Olive Manufacturing Wastewater Treatment Using the Peroxymonosulfate/Fe(III) System. Catalysts. 2024; 14(2):121. https://doi.org/10.3390/catal14020121
Chicago/Turabian StyleRivas, Francisco Javier, Fernando J. Beltrán, and Olga Gimeno. 2024. "Table Olive Manufacturing Wastewater Treatment Using the Peroxymonosulfate/Fe(III) System" Catalysts 14, no. 2: 121. https://doi.org/10.3390/catal14020121