- Article
Ligand Screening for Enzyme Immobilization Enables Efficient Removal of Aflatoxin B1 in Continuous Flow System
- Yujie Peng,
- Shenglong Mu and
- Jun Ge
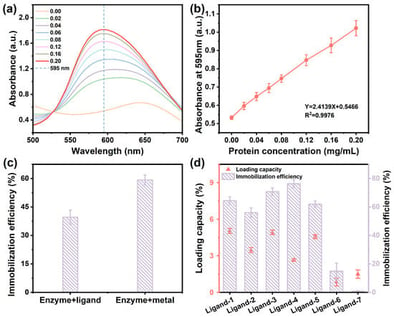
Aflatoxin B1 (AFB1) contamination is a significant issue for the safety of edible oils. Biodegradation of mycotoxins represents a green and efficient approach. However, enzymes exhibit low catalytic activity and stability under harsh conditions, leading to rapid deactivation in edible oils. Zeolitic imidazolate frameworks (ZIFs) possess high specific surface areas, tunable pore sizes, and excellent thermal stability. Immobilizing enzymes on ZIFs can address the problem of enzyme inactivation during application. Although the stability of the enzyme can be enhanced after immobilization, the overall enzymatic activity remains limited. To overcome the issues of low catalytic activity and poor cycling stability associated with enzymes immobilized on ZIF-8 using 2-methylimidazole (2-mIM) as the ligand, this study optimized the ZIF structure through a ligand screening strategy. Both encapsulation efficiency and cycling stability were enhanced. This research found that the activity of Lac@ZIFs(IM), which uses imidazole (IM) as the ligand, was 2.16 times that of Lac@ZIF-8. The degradation efficiency of AFB1 reached 93% within 4 h in edible oil using Lac@ZIFs(IM) as the catalyst, which was 21-fold higher than that of free laccase. Lac@ZIFs(IM) exhibited excellent activity in the continuous flow system. After 20 h of continuous reaction, the activity of Lac@ZIFs(IM) was 6.6 times that of Lac@ZIF-8. This study provides a novel approach for the efficient enzymatic degradation of mycotoxins.
12 February 2026