- Article
Green Synthesis of Silver-Magnetite Co-Decorated Acrylic Fabrics Using Brachychiton populneus Extract for Antimicrobial and Antioxidant Applications
- Rasha A. Zailaee,
- Reda M. El-Shishtawy and
- Yaaser Q. Almulaiky
- + 2 authors
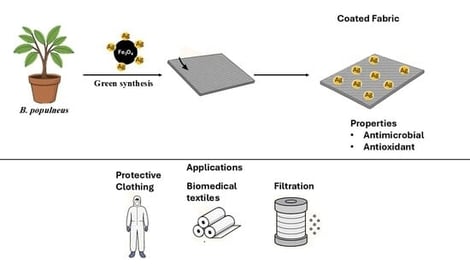
This manuscript reports a green approach for producing multifunctional acrylic fabrics co-decorated with Fe3O4 and Ag nanoparticles using Brachychiton populneus extract. Acrylic fabric was first amidoxime-functionalized to enable strong anchoring of Fe3O4 nanoparticles, followed by in situ deposition of AgNPs, during which the extract’s phytochemicals acted as reducing and stabilizing agents. FTIR, SEM/EDX, and VSM analyses confirmed successful surface modification and nanoparticle incorporation. The sequential treatments produced measurable add-on values (16.7% after amidoximation, followed by 10.9% and 8.5% after Fe3O4 and AgNP deposition, respectively). The Ag/Fe3O4-coated fabrics exhibited enhanced hydrophobicity and strong antimicrobial activity, with inhibition zones up to 14 mm against bacteria (including MRSA) and 26.9 mm against fungi at the highest Ag loading. Antioxidant activity was also markedly improved, showing up to a 78-fold increase in reducing power. Overall, this sustainable plant-mediated route provides an effective strategy for developing antimicrobial and antioxidant acrylic textiles for technical and protective applications.
2 February 2026