- Review
The Role of Metabolites in Acyclovir-Induced Neurotoxicity and Nephrotoxicity
- Asma Aboelezz and
- Sherif Hanafy Mahmoud
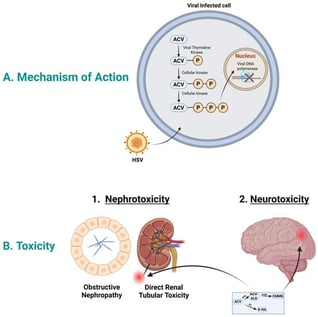
Acyclovir is an antiviral drug effective against infections caused by herpes simplex and varicella zoster viruses. It is given intravenously to treat serious infections such as herpes encephalitis. High acyclovir concentrations could cause toxicity, observed mainly as nephrotoxicity and, to a lesser extent, neurotoxicity. Acyclovir nephrotoxicity is primarily attributed to the crystallization of acyclovir within the renal tubules, although additional mechanisms may also contribute. However, the mechanism of acyclovir-induced neurotoxicity is unknown. Acyclovir is mainly eliminated from the body through renal excretion; however, around 15–20% of acyclovir is metabolized subsequently by alcohol and aldehyde dehydrogenase to the main metabolite 9-carboxymethoxymethylguanine (CMMG), and around 2% is metabolized by aldehyde oxidase to the minor metabolite, 8-hydroxyl acyclovir. It has been suggested that CMMG levels above 10 µmol/mL in the serum and 1 µmol/mL in the cerebrospinal fluid are highly associated with neurotoxicity. Studies have shown that there is a potential contribution of CMMG to acyclovir-induced neurotoxicity and of the acyclovir aldehyde to nephrotoxicity. In this narrative review, we approach the topic of acyclovir metabolites and their association with acyclovir toxicity. Moreover, we identify the research gap of the mechanisms by which these metabolites contribute to toxicity.
2 February 2026