- Review
Multi-Omics Approaches to Understanding Therapy Resistance in Acute Lymphoblastic Leukemia
- Xiuyun Wu and
- Jingxin Zhang
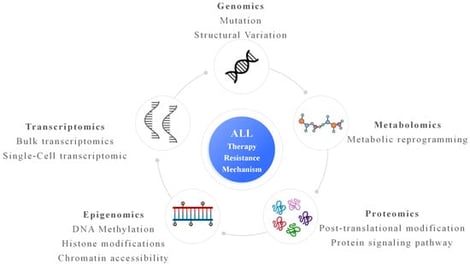
Therapy resistance remains a major cause of relapse and poor outcomes in acute lymphoblastic leukemia (ALL). Recent multi-omics studies in ALL have revealed that resistance arises from a combination of leukemia-specific genetic lesions, treatment-driven clonal evolution, and adaptive non-genetic programs. Genomic analyses have identified recurrent alterations associated with resistance to chemotherapy, tyrosine kinase inhibitors, and immunotherapies, while single-cell profiling has uncovered heterogeneous cell states that persist during treatment and contribute to minimal residual disease. Emerging epigenetic, proteomic, and metabolic data further indicate that reversible regulatory and signaling changes play a central role in leukemic persistence. Integrative analyses are beginning to define convergent resistance pathways and clinically relevant biomarkers, although longitudinal sampling and clinical translation remain limited. This review summarizes the current multi-omics landscape of therapy resistance in ALL and discusses opportunities to improve risk stratification and therapeutic strategies.
29 January 2026