- Article
Functional State of Lampbrush Chromosomes in Early Vitellogenic Oocytes of Hibernating Frogs Rana temporaria
- Nadya V. Ilicheva and
- Olga I. Podgornaya
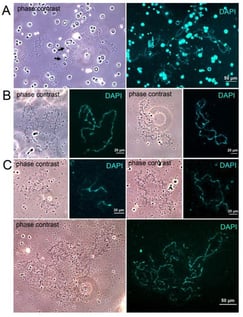
Lampbrush chromosomes (LBCs) are a feature of amphibian oocytes and are typically associated with high levels of transcription during active oocyte growth. However, their state during winter hibernation has not been studied. Here, we investigated LBCs in early vitellogenic oocytes (early stage 4) of the grass frog Rana temporaria during winter hibernation. We found that the chromosomes retained their lampbrush morphology, and the phosphorylated form of RNA polymerase II resided on the lateral loops. Transcription on the lateral loops was reduced but detectable at cold conditions and significantly increased when the oocytes were transferred at room temperature. Satellite S1a transcripts were detected at the lateral loops of the chromosomes by RNA FISH. The possible significance of maintaining chromosomes in the lampbrush form during hibernation is discussed.
2 February 2026