- Review
Complement at the Nano–Neuroimmune Interface: A Hypothesis-Driven Perspective on Opioid Use Disorder
- Alexander Jacob,
- Harbir Singh and
- Jessy J. Alexander
- + 5 authors
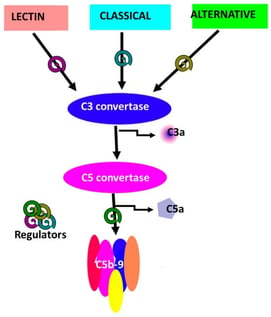
The complement system is a central component of innate immunity with established roles in host defense and emerging functions in neurodevelopment, synaptic remodeling, and neuroimmune communication within the central nervous system (CNS). In parallel, advances in nanotechnology have not only enabled targeted strategies for CNS drug delivery but have also revealed that many nanomaterials interact with and activate complement, influencing biodistribution, safety, and inflammatory responses. Opioid use disorder (OUD) is increasingly recognized as a condition associated with chronic neuroimmune dysregulation involving glial activation, altered cytokine signaling, and blood–brain barrier (BBB) disruption. However, direct experimental or clinical measurements of complement activation in OUD remain limited. Current evidence linking complement pathways to opioid exposure is derived largely from indirect observations, including transcriptomic alterations, glial phenotypes, and inflammatory signatures in preclinical and translational models, which collectively suggest, but do not yet definitively establish, complement involvement in opioid-induced neuroimmune signaling. This review synthesizes current knowledge at the intersection of complement biology, nanomedicine, and opioid-associated neuroimmune changes. It distinguishes well-established mechanisms of complement activation by nanomaterials from emerging and inferential evidence linking complement signaling to opioid exposure. This hypothesis-generating framework integrates complement signaling with opioid receptor and TLR4 pathways in glial and endothelial compartments, examining their potential protective and pathological CNS roles while outlining the translational promise and current evidence gaps of complement-aware nanotechnologies for addiction neuroscience.
13 February 2026