- Systematic Review
Efficacy and Safety of Minocycline-Containing Bismuth Quadruple Therapies Versus Standard First-Line Bismuth Quadruple Therapies for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis
- Hakim Ullah Wazir,
- Abdul Muqeet Khuram and
- Ussama Shafaqat
- + 10 authors
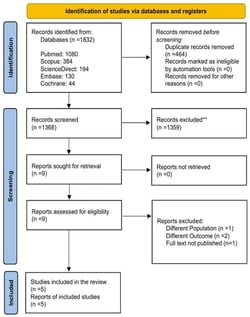
Background: Growing antibiotic resistance and the limited availability of key components in standard Helicobacter pylori treatments have driven the search for effective alternatives. Minocycline, with its broad-spectrum activity and favorable pharmacokinetics, has emerged as a promising substitute. This meta-analysis compares the safety and efficacy of minocycline-containing bismuth quadruple therapy (MBQT) to conventional first-line BQT regimens, incorporating data from the recent study by Lin et al. Methods: The inclusion criteria were randomized controlled trials (RCTs) with a target population of both treatment-naïve and previously treated patients diagnosed with Helicobacter pylori (H. pylori) infection. The intervention received by eligible patients was a minocycline–bismuth quadruple therapy (MBQT) regimen containing bismuth, minocycline, proton pump inhibitors (PPI), and any additional antibiotic with a minimum period of 2 weeks of administration. We excluded study designs other than RCT and clinical trials that include patients without confirmed H. pylori infection, animal populations, in vitro experiments, and reports of other outcomes that did not include a minimum intervention duration of 2 weeks. A comprehensive literature search was conducted on PubMed, EMBASE, Cochrane Library, and ScienceDirect from inception to 20 May 2025. After screening via Rayyan, data were extracted on an Excel spreadsheet. Quality was assessed using the Cochrane RoB 2.0 tool. Eligible randomized controlled trials (RCTs) were included and analyzed using RevMan 5.4. Outcomes assessed were intention-to-treat and per-protocol eradication rates. Adverse effects were compared among therapies. A random-effects model was used; an I2 < 50% and p-value < 0.05 indicated homogeneity and significant results respectively. Results: Five RCTs with 7 interventions involving 2812 patients were included. The pooled odds ratio (OR) for MBQT in intention-to-treat (ITT) analysis was 1.25 (95% CI: 0.96–1.61), showing a non-significant trend. No heterogeneity was detected (I2 = 0.0%). In the modified ITT (mITT) analysis (2 studies), MBQT showed higher eradication (OR: 1.70, 95% CI: 0.00–1042.90), but wide CI and high heterogeneity (I2 = 70.7%) limited interpretation. All studies were included in the per-protocol (PP) analysis, which showed a statistically significant improvement with MBQT (OR: 1.67, 95% CI: 1.14–2.45) and low heterogeneity (I2 = 5.2%), suggesting consistent results. Although not statistically significant, MBQT was associated with a slightly lower rate of adverse events compared to standard therapy (OR: 0.81, 95% CI: 0.59–1.12). I2 = 50.6% showed moderate heterogeneity in safety outcomes. Discussion: the number of included RCTs was modest, with only five studies meeting eligibility criteria, and only two contributing to the modified intention-to-treat analysis. The risk-of-bias assessment showed variation in methodological quality across the included studies. Several studies exhibited high risk judgments in critical domains. particularly randomization, deviations from intervention, and selective reporting. Patients who completed the treatment benefited more from MBQT, which also had a comparable safety profile to conventional BQT regimens. In the treatment of H. pylori infection, MBQT may be considered a safe alternative for first-line treatment.
6 February 2026