- Review
A Review of Patient-Reported Outcomes and Clinical Outcomes in Acute and Chronic Myeloid and Lymphoid Leukemias
- Bryan Chan,
- Eesha Balar and
- Catherine Lai
- + 3 authors
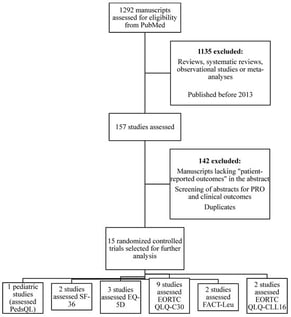
Introduction: This review specifically focuses on interventional clinical trials in leukemias and myelodysplastic syndromes (MDS), summarizing how patient-reported outcome measures (PROMs) have been implemented to evaluate treatment effects rather than to directly influence clinical outcomes. Objective: Clinical outcomes of interest typically include response rates, disease-free survival (DFS), and overall survival (OS). Patient-reported outcome measures (PROMs) are standardized questionnaires that collect information regarding health outcomes directly from the patient and are used to evaluate new treatments and healthcare quality. In addition, the use of PROMs in cancer care has been shown to improve patient-provider communication and patient satisfaction. Material and Methods: This is a qualitative, narrative synthesis and review structured around PROMs focused on six critical themes: symptoms/symptom burden, physical, emotional, social/role, and functional status, and global health status measurement. Results: PROMs that are assessed in oncologic research include the EORTC QLQ-C30, FACT-Leu, QLQ-CLL16, and EQ-5D. PROs are associated with clinical outcomes such as DFS and OS, and the FACT-Leu scales, HRQOL and physical functioning scores were independent prognosticators of OS in patients with AML. Conclusions: Through our review, notable trends were identified that further highlight the importance of greater incorporation of PRO measures in future clinical trials, particularly in the understudied realm of hematologic malignancies, in order to better delineate the link between survival and HRQOL.
6 February 2026