- Case Report
A Perplexing Plexopathy After Pembrolizumab Therapy in Early-Stage Triple-Negative Breast Cancer
- Toluwalogo Baiyewun,
- Brian McNamara and
- Seamus O’Reilly
- + 6 authors
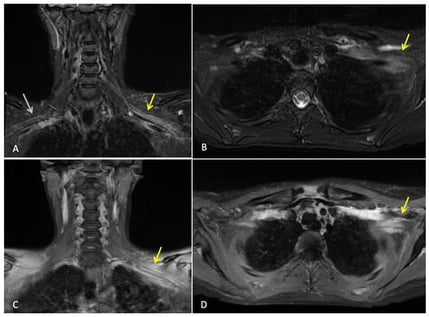
Background: In triple-negative breast cancer (TNBC), the addition of immunotherapy has significantly improved outcomes. Immune-related adverse events (irAEs) can be accelerated in patients with pre-existing autoimmune (AI) conditions. The treatment-response standardized protocol used in clinical care raises concerns about the need for right-sizing strategies. As the use of immunotherapy expands, recognizing toxicity from recurrence and optimizing response-adapted approaches are essential to balance cure with quality of survival. Case Presentation: A 38-year-old pregnant woman with a distant history of uveitis and psoriasis was discovered to have pregnancy-associated TNBC. Postnatally, she was treated with neoadjuvant chemotherapy and pembrolizumab, followed by wire-guided left breast wide local excision and sentinel lymph node biopsy of the left axilla. After surgery, residual cancer was noted. She continued adjuvant pembrolizumab and adjuvant radiotherapy 40.05 Gy/15 fr to the breast and nodes, followed by a 13.35 Gy/5 fr boost to the tumour bed (breast). Despite a persistent residual tumour, pembrolizumab was continued as per protocol in a response-agnostic manner. At the end of one year of adjuvant pembrolizumab, she developed progressive numbness and weakness in the ipsilateral arm, initially raising suspicion for local recurrence. Comprehensive MRI and PET-CT imaging did not identify recurrent tumour or new metastatic disease. Electromyography confirmed a lower-trunk brachial plexopathy without a structural cause. An immune-mediated process was diagnosed by a process of elimination. Despite treatment with 1st-line high-dose corticosteroids and 2nd-line intravenous immunoglobulin (IVIG), improvement was limited. Therapeutic plasmapheresis led to marked functional recovery and symptom resolution 20 months later. Discussion: Four main challenges are identified: (1) the diagnostic difficulty in identifying local recurrence or radiation injury from immune-related neuropathy; (2) the emerging therapeutic role of plasmapheresis in steroid-refractory irAEs; (3) the possible inconsistencies between rare toxicities observed in clinical trials vs. clinical practice; and (4) the limitations in response in adjuvant therapy, particularly in patients with coexisting AI conditions. Conclusions: Early recognition and accurate distinction from tumour recurrence, as well as support for plasmapheresis as a potential option in steroid-refractory presentations, have been shown to improve patient survival and symptom reduction. With increasing use of immunotherapy, real-world toxicity data, predictive biomarkers, and personalized treatment strategies are urgently needed to balance cure with long-term functional outcomes.
20 February 2026