- Review
The Role of Raf Kinase Inhibitor Protein (RKIP) in HER2+ Breast Cancer Immune Evasion
- Ania Khachikian,
- Mai Ho and
- Benjamin Bonavida
Breast cancer (BC) is a prevalent malignancy worldwide among women. HER2 overexpression in a subset of BC (HER2+ BC) serves as a critical oncogenic driver and contributes to immune evasion. The Raf Kinase Inhibitor Protein (RKIP), a metastasis suppressor and an immune enhancer, is underexpressed in HER2+ BC. The treatment of HER2+ BC with anti-HER2 mAbs or chemical inhibitors has resulted in significant clinical responses in a subset of patients; however, unresponsiveness in a larger subset was due to acquired and induced resistance. These findings highlight the need for the development of new effective therapies. By analyzing the signaling pathways mediated by both RKIP and HER2 in HER2+ BC, we have found that RKIP and HER2 downstream signaling and inductions showed an inverse relationship. These suggested the presence of a dysregulated RKIP-HER2 axis in HER2+ BC mediating immune evasion. These findings were corroborated by bioinformatic analyses. The immune evasion induced by the overexpression of HER2 was due, in part, to its regulation of the expression of PD-L1, the polarization of TAMs, the infiltration of suppressor cells (Tregs, MDSCs), and the inhibition of anti-tumor CD8+ T cells, resulting in an overall immunosuppressive TME. In contrast, RKIP expression inhibits critical signaling pathways that regulate HER2 expression, including the Raf-MEK-ERK, NF-kB, and PI3K/Akt pathways, thereby aborting HER2-mediated mechanisms of immune evasion. Overall, we analyzed the cross-talk signaling pathways between RKIP and HER2, established a novel dysregulated axis in HER2+ BC, and delineated the various mechanisms involved in the regulation of immune evasion by RKIP and HER2. Hence, we present various therapeutic strategies aimed at targeting the RKIP-HER2 axis in HER2+ BC to circumvent unresponsiveness to therapeutics and immune evasion.
8 February 2026






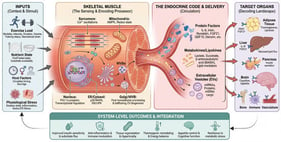
![RKIP and HER2 cross-talk signaling. The MAPK, NF-κB, and Akt pathways regulated by HER2 expression are shown (⇢). RKIP blocks critical signaling pathways, such as the MAPK, NF-κB, and PI3K/Akt pathways () [144,145,146,147]. However, by inhibiting these signaling cascades, RKIP reduces HER2-related signaling [12,133,134,135,136]. This figure demonstrates the antagonistic relationship between RKIP and HER2 and the regulatory feedback loop they form. Additionally, it illustrates three common mechanisms by which RKIP and HER2 interact, influencing cancer treatment and HER2-mediated cell proliferation. Created with BioRender.com. Accessed on 1 November 2025.](https://mdpi-res.com/cdn-cgi/image/w=470,h=317/https://mdpi-res.com/cells/cells-15-00319/article_deploy/html/images/cells-15-00319-g001-550.jpg)