- Article
Multi-Institutional CT Scan-Based Radiomics for Predicting Tumor PD-L1 Expression in Patients with Advanced and Limited Non-Small Cell Lung Cancer
- Ralph Saber,
- Marion Tonneau and
- Bertrand Routy
- + 8 authors
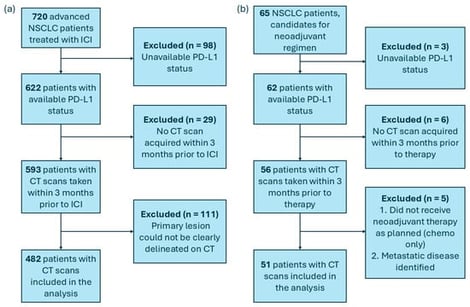
Background/Objectives: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment landscape of advanced non-small cell lung cancer (NSCLC), yet 70% of patients experience disease progression, underscoring the critical need for predictive biomarkers. Programmed death-ligand 1 (PD-L1) expression remains the most adopted biomarker for ICIs. With the emergence of machine learning, the development of radiomics algorithms based on CT scan images has demonstrated potential as a novel addition to the biomarker landscape in oncology. In this study, we aimed to develop a non-invasive surrogate of PD-L1 expression (rad-PDL1) derived from computed tomography (CT) scan imaging and compare its predictive value to pathological assessments. Furthermore, we evaluated its generalizability across advanced and limited-stage NSCLC. Methods: Radiomics features extracted from pretreatment CT were analyzed using a self-training pipeline that incorporated the feature tokenizer Transformer model to classify tumors as high vs. low PD-L1 expression. We included 482 advanced NSCLC patients treated with ICIs across three medical centers who were divided into training and hold-out validation sets. The algorithm was then further validated in an independent cohort of 51 patients with limited NSCLC treated with neoadjuvant ICI and chemotherapy. Results: Our pipeline demonstrated strong predictive performance in primary and independent validation (AUC = 0.75 and 0.68, accuracy = 0.73 and 0.69, respectively), highlighting its generalizability and adaptability to various disease stages. Kaplan–Meier curves revealed a longer progression-free survival for patients in the high rad-PDL1. Conclusions: These results demonstrate the feasibility of a CT-based radiomic surrogate of PD-L1 expression, showing partial generalization to an independent neoadjuvant cohort, while highlighting the need for larger prospective multi-site validation before clinical implementation.
8 February 2026