The Impact of Different Self-Selected Walking Speeds on Muscle Synergies in Transfemoral Amputees during Transient-State Gait
Abstract
1. Introduction
1.1. Muscle Synergy Analysis at Different Speeds
1.2. Muscle Synergy in the Pathological Population
1.3. Muscle Synergy in Lower Limb Amputees
1.4. Aims and Objectives
2. Materials and Methods
2.1. Subjects
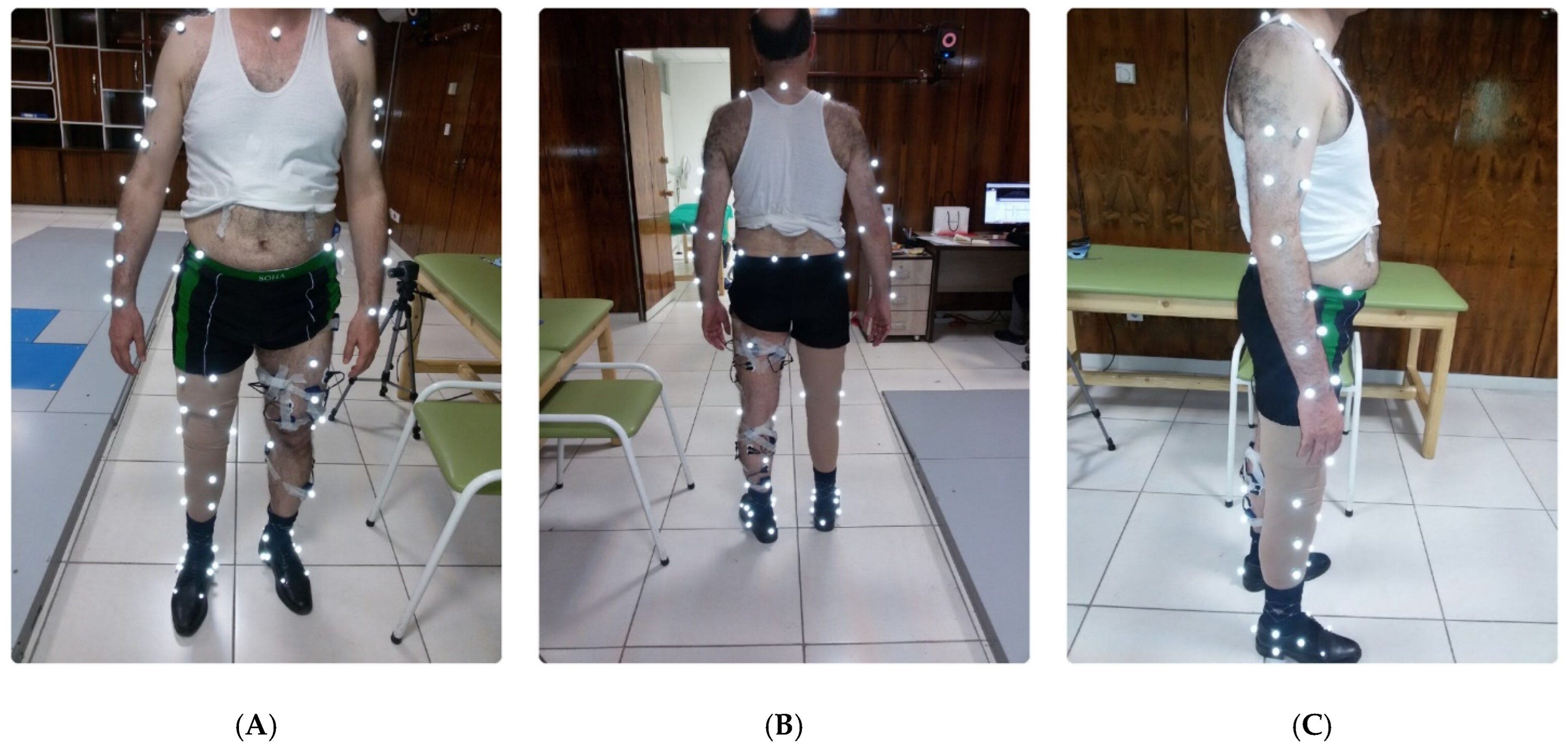
2.2. Experimental Protocol
2.3. Data Collection
2.4. Processing Surface EMG and Preparation for Muscle Synergy Analysis
2.5. Concatenated Non-Negative Matrix Factorization (CNMF) Frameworks
2.6. CNMF MATLAB Implementation
2.7. Dimensionality (Variance Accounted for)
2.8. Synergy Output Normalization
2.9. Between Population Synergy Sorting
2.10. Statistical Analysis
3. Results
3.1. Dimensionality
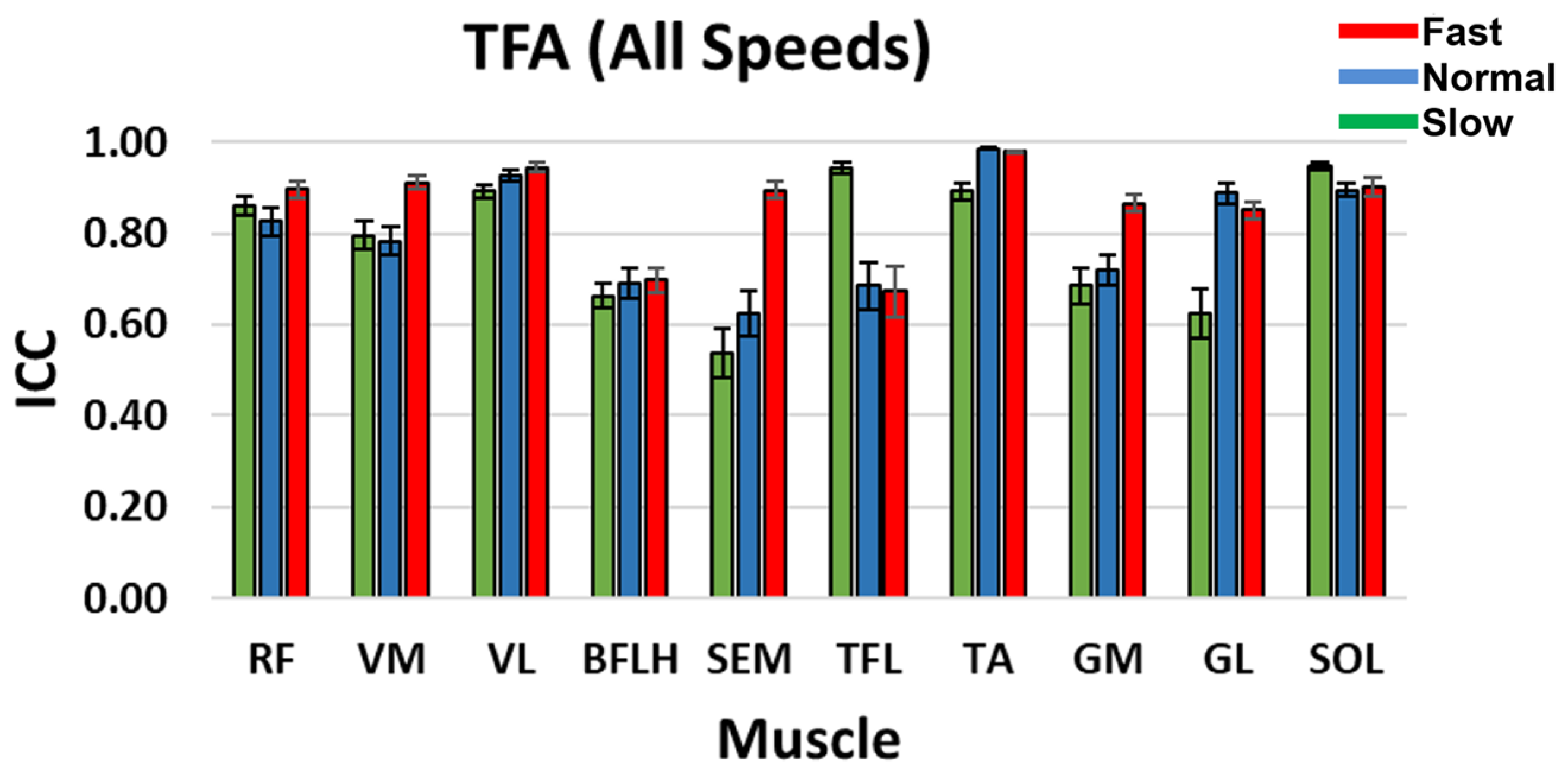
3.2. Intra-Class Correlation Coefficient (ICC)
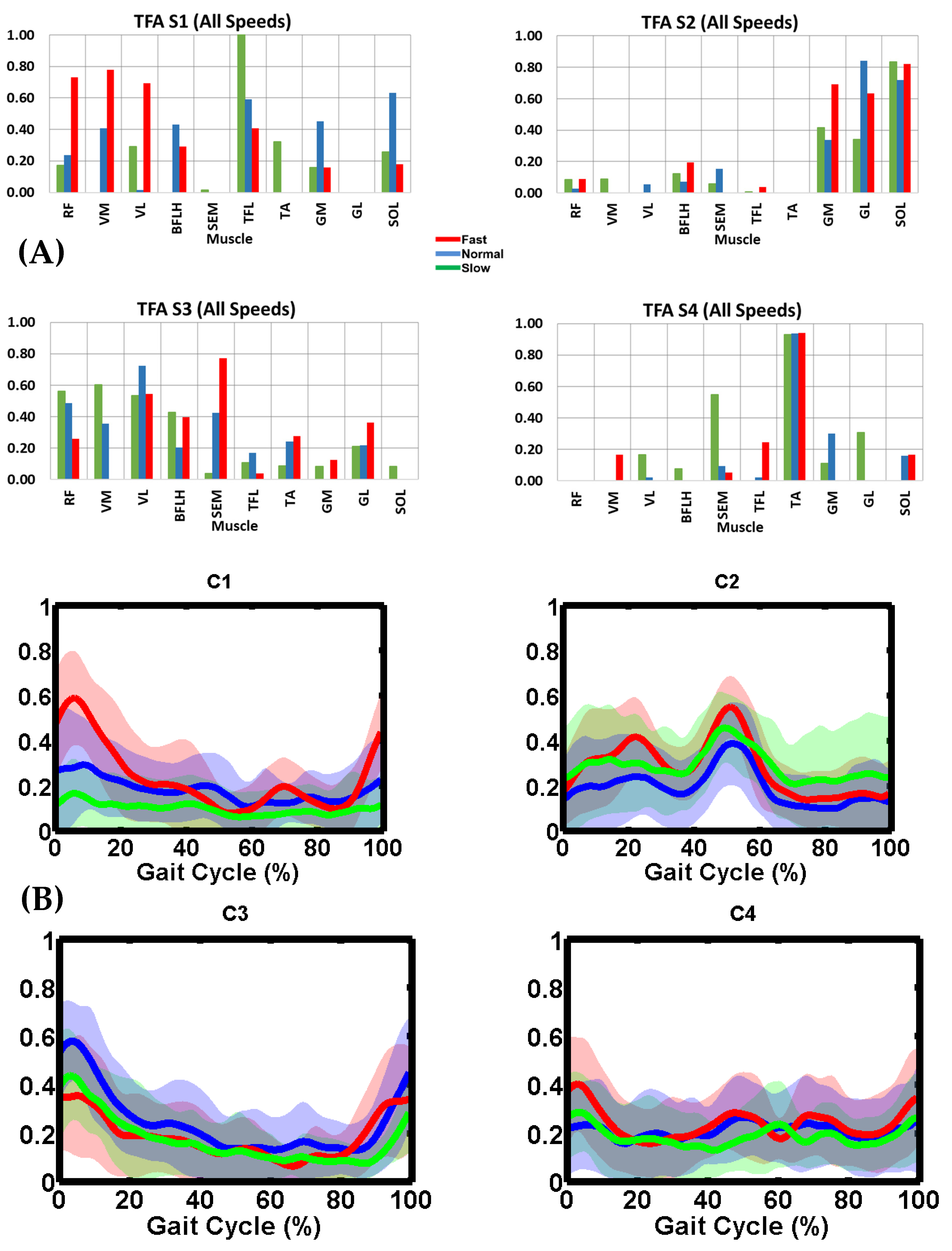
3.3. Inter-Subject Variability of C
3.4. TFA Muscle Synergy Description
3.5. Synergy Vector Comparison
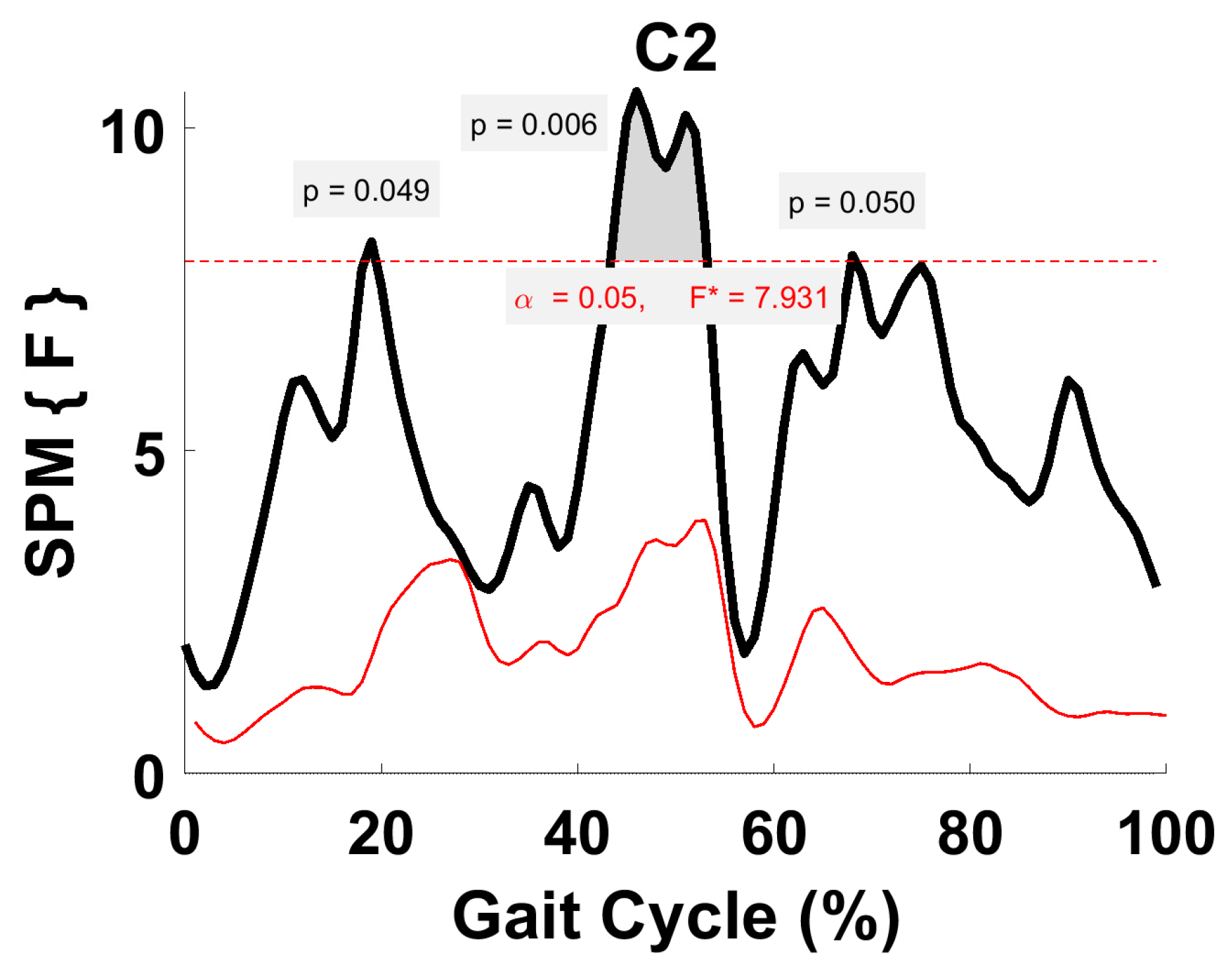
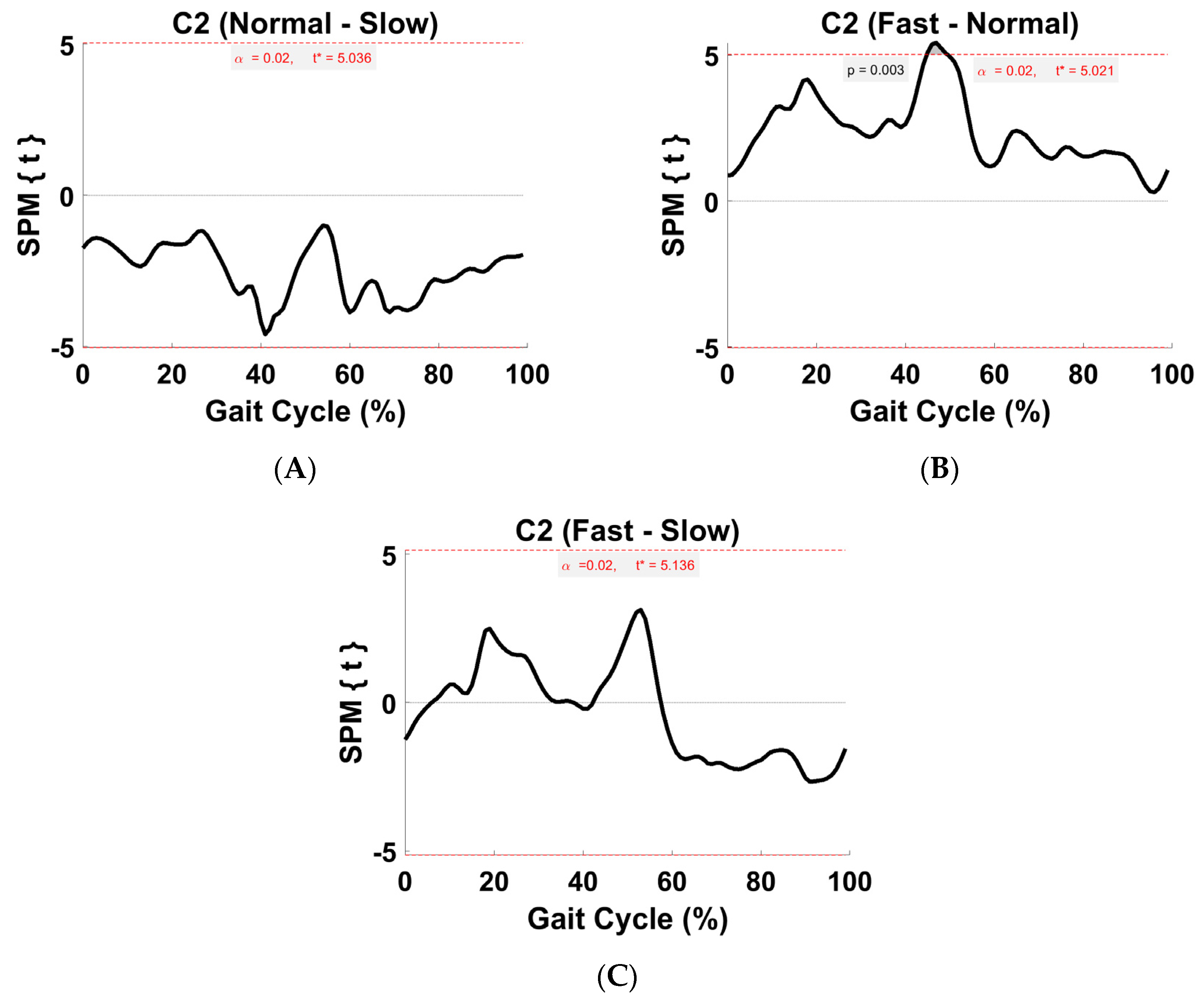
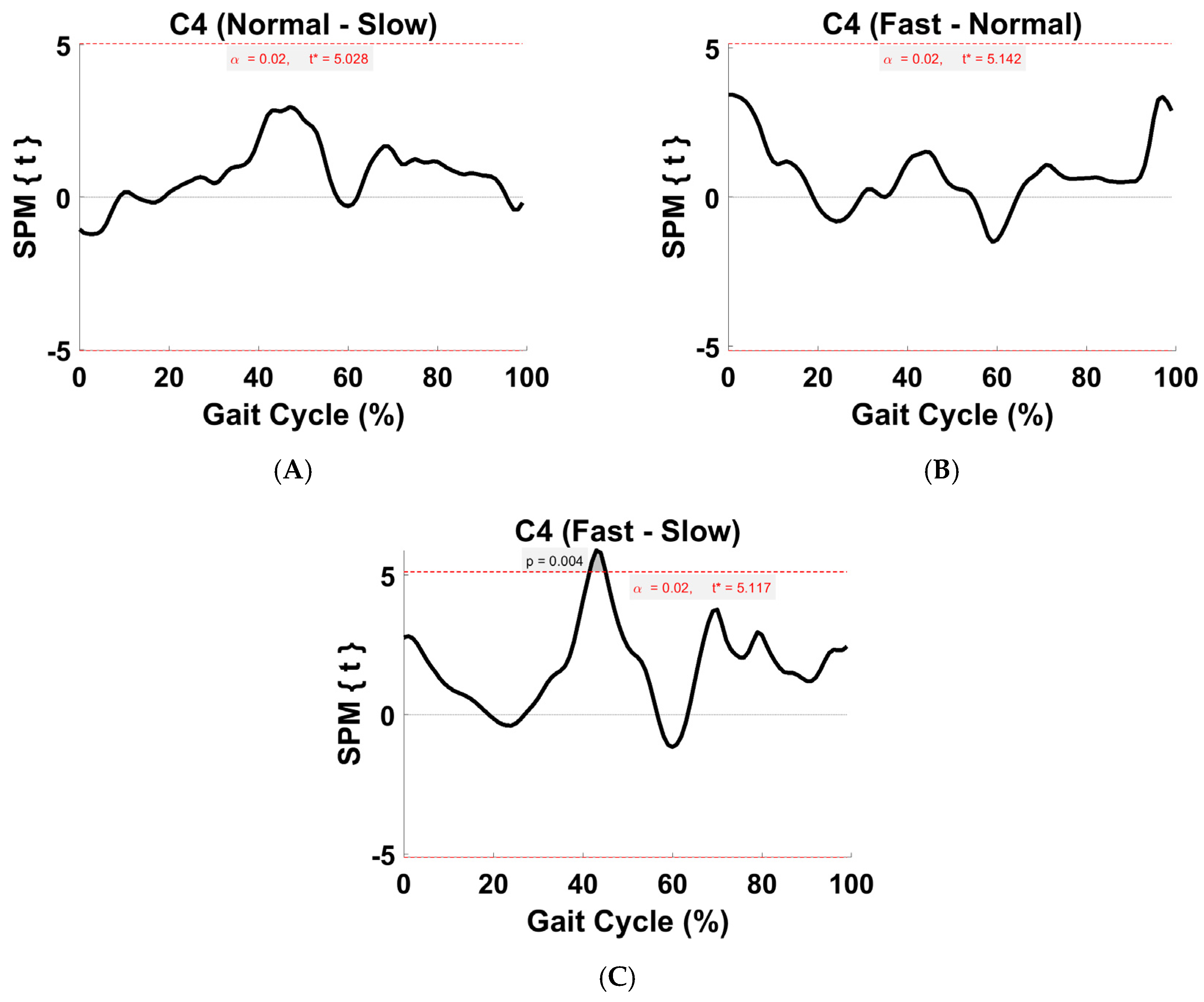
3.6. Activation Coefficient Profile Comparison
4. Discussion
4.1. VAF
4.2. CNMF Activation Coefficient Profile Repeatability (ICC)
4.3. Within-Subject Comparison (Synergy/Module/S)
4.4. Synergy Vector Comparison with Literature
4.5. Within-Subject Comparison (Activation Coefficient Profiles)
4.6. Comparison of the Activation Coefficient Profile with Literature
4.7. Potential Rehabilitative and Assistive Applications
4.8. Limitations
5. Summary
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor patterns in human walking and running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef]
- Mehryar, P.; Shourijeh, M.S.; Rezaeian, T.; Khandan, A.R.; Messenger, N.; O’Connor, R.; Farahmand, F.; Dehghani-Sanij, A. Muscular activity comparison between non-amputees and transfemoral amputees during normal transient-state walking speed. Med. Eng. Phys. 2021, 95, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Mehryar, P.; Rezaeian, T.; Shourijeh, M.S.; Raveendranathan, V.; Messenger, N.; O’Connor, R.; Dehghani-Sanij, A. Changes in knee joint kinetics of transfemoral amputee’s intact leg: An osteoarthritis indication? Gait Posture 2017, 57, 151–152. [Google Scholar] [CrossRef]
- Mehryar, P.; Shourijeh, M.S.; Rezaeian, T.; Khandan, A.R.; Messenger, N.; O’Connor, R.; Farahmand, F.; Dehghani-Sanij, A. Differences in muscle synergies between healthy subjects and transfemoral amputees during normal transient-state walking speed. Gait Posture 2020, 76, 98–103. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, C.; Ranaldi, S.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Lacquaniti, F.; Conforto, S. Modular motor control of the sound limb in gait of people with trans-femoral amputation. J. Neuroeng. Rehabil. 2019, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, C.D.; Sanderson, D.J.; Inglis, J.T.; Chua, R. Postural and movement adaptations by individuals with a unilateral below-knee amputation during gait initiation. Gait Posture 2003, 18, 158–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, S.; Choi, H.; Ryu, K.; Kim, S.; Kim, Y. Kinematics, kinetics and muscle activities of the lower extremity during the first four steps from gait initiation to the steady-state walking. J. Mech. Sci. Technol. 2009, 23, 204–211. [Google Scholar] [CrossRef]
- Mehryar, P. High Dimensional Surface Electromyography and Low Dimensional Muscle Synergy in Lower Limb Amputees during Transient-and Steady-State Gait. Doctoral Thesis, University of Leeds, Leeds, UK, 2018. [Google Scholar]
- Mbourou, G.A.; Lajoie, Y.; Teasdale, N. Step length variability at gait initiation in elderly fallers and non-fallers, and young adults. Gerontology 2003, 49, 21–26. [Google Scholar] [CrossRef]
- Wong, C.K.; Chihuri, S.T.; Li, G. Risk of fall-related injury in people with lower limb amputations: A prospective cohort study. J. Rehabil. Med. 2016, 48, 80–85. [Google Scholar] [CrossRef]
- Vanicek, N.; Strike, S.; McNaughton, L.; Polman, R. Gait patterns in transtibial amputee fallers vs. non-fallers: Biomechanical differences during level walking. Gait Posture 2009, 29, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vargas, J.; Sartori, M.; Dosen, S.; Torricelli, D.; Pons, J.L.; Farina, D. A predictive model of muscle excitations based on muscle modularity for a large repertoire of human locomotion conditions. Front. Comput. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef][Green Version]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2009, 103, 844–857. [Google Scholar] [CrossRef]
- Gui, K.; Zhang, D. Influence of locomotion speed on biomechanical subtask and muscle synergy. J. Electromyogr. Kinesiol. 2016, 30, 209–215. [Google Scholar] [CrossRef]
- Ivanenko, Y.; Cappellini, G.; Poppele, R.; Lacquaniti, F. Spatiotemporal organization of α-motoneuron activity in the human spinal cord during different gaits and gait transitions. Eur. J. Neurosci. 2008, 27, 3351–3368. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Tomita, A.; Ando, R.; Watanabe, K.; Akima, H. Similarity of muscle synergies extracted from the lower limb including the deep muscles between level and uphill treadmill walking. Gait Posture 2018, 59, 134–139. [Google Scholar] [CrossRef]
- Hagio, S.; Fukuda, M.; Kouzaki, M. Identification of muscle synergies associated with gait transition in humans. Front. Hum. Neurosci. 2015, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef]
- McGowan, C.P.; Neptune, R.R.; Clark, D.J.; Kautz, S.A. Modular control of human walking: Adaptations to altered mechanical demands. J. Biomech. 2010, 43, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, F.; Ivanenko, Y.P.; Zago, M. Patterned control of human locomotion. J. Physiol. 2012, 590, 2189–2199. [Google Scholar] [CrossRef]
- Kibushi, B.; Hagio, S.; Moritani, T.; Kouzaki, M. Speed-dependent modulation of muscle activity based on muscle synergies during treadmill walking. Front. Hum. Neurosci. 2018, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Ogawa, T.; Kawashima, N.; Shinya, M.; Nakazawa, K. Distinct sets of locomotor modules control the speed and modes of human locomotion. Sci. Rep. 2016, 6, 36275. [Google Scholar] [CrossRef]
- Rodriguez, K.L.; Roemmich, R.T.; Cam, B.; Fregly, B.J.; Hass, C.J. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 2013, 124, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.M.; Hofstoetter, U.S.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Human spinal locomotor control is based on flexibly organized burst generators. Brain 2015, 138, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; Rozumalski, A.; Schwartz, M.H. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev. Med. Child Neurol. 2015, 57, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef]
- Cheung, V.C.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef]
- Hayes, H.B.; Chvatal, S.A.; French, M.A.; Ting, L.H.; Trumbower, R.D. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin. Neurophysiol. 2014, 125, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef]
- Gizzi, L.; Nielsen, J.F.; Felici, F.; Ivanenko, Y.P.; Farina, D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J. Neurophysiol. 2011, 106, 202–210. [Google Scholar] [CrossRef]
- Mehryar, P.; Shourijeh, M.; Rezaeian, T.; Iqbal, N.; Messenger, N.; Dehghani-Sanij, A.A. Changes in synergy of transtibial amputee during gait: A pilot study. In Biomedical & Health Informatics (BHI), Proceedings of the 2017 IEEE EMBS International Conference, Orlando, FL, USA, 16–19 February 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 325–328. [Google Scholar]
- Mehryar, P.; Shourijeh, M.; Maqbool, H.F.; Torabi, M.; Dehghani-Sanij, A.A. Muscle synergy analysis in transtibial amputee during ramp ascending activity. In Engineering in Medicine and Biology Society (EMBC), Proceedings of the 2016 IEEE 38th Annual International Conference, Orlando, FL, USA, 16–20 August 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1676–1679. [Google Scholar]
- Mehryar, P.; Shourijeh, M.S.; Dehghani-Sanij, A.A. Muscle Synergy Analysis in Transtibial Amputee during Ramp Descending Activity; Springer International Publishing: Cham, Switzerland, 2017; pp. 945–950. [Google Scholar]
- Bus, S.A.; de Lange, A. A comparison of the 1-step, 2-step, and 3-step protocols for obtaining barefoot plantar pressure data in the diabetic neuropathic foot. Clin. Biomech. 2005, 20, 892–899. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede Roessingh Res. Dev. 2007, 10, 108–112. [Google Scholar]
- Frère, J. Spectral properties of multiple myoelectric signals: New insights into the neural origin of muscle synergies. Neuroscience 2017, 355, 22–35. [Google Scholar] [CrossRef]
- Neptune, R.R.; Clark, D.J.; Kautz, S.A. Modular control of human walking: A simulation study. J. Biomech. 2009, 42, 1282–1287. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Gizzi, L.; Farina, D.; Kersting, U.G. Motor modules of human locomotion: Influence of EMG averaging, concatenation, and number of step cycles. Front. Hum. Neurosci. 2014, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Serrancolí, G.; Monllau, J.C.; Font-Llagunes, J.M. Analysis of muscle synergies and activation–deactivation patterns in subjects with anterior cruciate ligament deficiency during walking. Clin. Biomech. 2016, 31, 65–73. [Google Scholar] [CrossRef]
- Shourijeh, M.S.; Flaxman, T.E.; Benoit, D.L. An approach for improving repeatability and reliability of non-negative matrix factorization for muscle synergy analysis. J. Electromyogr. Kinesiol. 2016, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Torres-Oviedo, G.; Macpherson, J.M.; Ting, L.H. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 2006, 96, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Gizzi, L.; Lloyd, D.G.; Farina, D. A musculoskeletal model of human locomotion driven by a low dimensional set of impulsive excitation primitives. Front. Comput. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Ting, L.H. Muscle synergies characterizing human postural responses. J. Neurophysiol. 2007, 98, 2144–2156. [Google Scholar] [CrossRef]
- Portney, L.; Watkins, M. Foundations of Clinical Research: Application to Practice; Appleton & Lange: Stamford, CT, USA, 1993. [Google Scholar]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef]
- Guidetti, L.; Rivellini, G.; Figura, F. EMG patterns during running: Intra-and inter-individual variability. J. Electromyogr. Kinesiol. 1996, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hug, F. Can muscle coordination be precisely studied by surface electromyography? J. Electromyogr. Kinesiol. 2011, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miall, R.C. Walking the walk. Nat. Neurosci. 2007, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.A.; Vanrenterghem, J.; Pataky, T.C. Statistical Parametric Mapping (SPM) for alpha-based statistical analyses of multi-muscle EMG time-series. J. Electromyogr. Kinesiol. 2015, 25, 14–19. [Google Scholar] [CrossRef] [PubMed]
- De Groote, F.; Jonkers, I.; Duysens, J. Task constraints and minimization of muscle effort result in a small number of muscle synergies during gait. Front. Comput. Neurosci. 2014, 8, 115. [Google Scholar] [CrossRef]
- Abd, A.T.; Singh, R.E.; Iqbal, K.; White, G. A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation. Biomechanics 2021, 1, 253–263. [Google Scholar] [CrossRef]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef]






| TFA Intra-Class Correlation Coefficient | ||||
|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |
| Slow | 0.89 | 0.80 | 0.97 | 0.87 |
| Normal | 0.98 | 0.94 | 0.90 | 0.29 |
| Fast | 0.98 | 0.97 | 0.94 | 0.85 |
| TFA Synergy 1 (All Speeds) | |||
|---|---|---|---|
| Module | Muscle | Activation | |
| Primary (>0.5) * | Secondary (<0.5) * | ||
| Fast | VM, RF, VL | TFL, BFLH, SOL, GM | ES, TSW |
| Normal | SOL, TFL | GM, BFLH, VM, RF, VL | ES-MS, TS, TSW |
| Slow | TFL | TA, VL, SOL, RF, GM, SEM | IC-LR, MS |
| TFA Synergy 2 (All Speeds) | |||
|---|---|---|---|
| Module | Muscle | Activation | |
| Primary (>0.5) | Secondary (<0.5) | ||
| Fast | SOL, GM, GL | BFLH, RF, TFL | MS, TS |
| Normal | GL, SOL | GM, SEM, BFLH, VL, RF | MS, TS |
| Slow | SOL | GM, GL, BFLH, RF, VM, SEM | MS, TS |
| TFA Synergy 3 (All Speeds) | |||
|---|---|---|---|
| Module | Muscle | Activation | |
| Primary (>0.5) | Secondary (<0.5) | ||
| Fast | SEM, VL | BFLH, GL, TA, RF, GM, TFL | ES, MSW-TSW |
| Normal | VL | RF, SEM, VM, TA, GL, BFLH, TFL | IC-LR, TSW |
| Slow | VM, RF, VL | BFLH, GL, TFL, TA, GM, SOL, SEM | IC-LR, TSW |
| TFA Synergy 4 (All Speeds) | |||
|---|---|---|---|
| Module | Muscle | Activation | |
| Primary (>0.5) | Secondary (<0.5) | ||
| Fast | TA | TFL, VM, SOL, SEM | IC-LR, TS, ISW, TSW |
| Normal | TA | GM, SOL, SEM, VL, TFL | IC-LR, TS-PSW-ISW, TSW |
| Slow | TA, SEM | GL, VL, GM, BFLH | IC-LR, PSW-ISW, TSW |
| S1 | S2 | S3 | S4 | Overall Average | |
|---|---|---|---|---|---|
| Normal vs. Slow | 0 | 0.78 | 0.72 | 0.61 | 0.53 |
| Normal vs. Fast | 0.13 | 0.83 | 0.66 | 0.83 | 0.61 |
| Fast vs. Slow | 0 | 0.89 | 0.2 | 0.5 | 0.40 |
| Module Average | 0.04 | 0.83 | 0.53 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehryar, P.; Shourijeh, M.; Rezaeian, T.; Khandan, A.; Messenger, N.; O’Connor, R.; Farahmand, F.; Dehghani-Sanij, A. The Impact of Different Self-Selected Walking Speeds on Muscle Synergies in Transfemoral Amputees during Transient-State Gait. Biomechanics 2024, 4, 14-33. https://doi.org/10.3390/biomechanics4010002
Mehryar P, Shourijeh M, Rezaeian T, Khandan A, Messenger N, O’Connor R, Farahmand F, Dehghani-Sanij A. The Impact of Different Self-Selected Walking Speeds on Muscle Synergies in Transfemoral Amputees during Transient-State Gait. Biomechanics. 2024; 4(1):14-33. https://doi.org/10.3390/biomechanics4010002
Chicago/Turabian StyleMehryar, Pouyan, Mohammad Shourijeh, Tahmineh Rezaeian, Aminreza Khandan, Neil Messenger, Rory O’Connor, Farzam Farahmand, and Abbas Dehghani-Sanij. 2024. "The Impact of Different Self-Selected Walking Speeds on Muscle Synergies in Transfemoral Amputees during Transient-State Gait" Biomechanics 4, no. 1: 14-33. https://doi.org/10.3390/biomechanics4010002
APA StyleMehryar, P., Shourijeh, M., Rezaeian, T., Khandan, A., Messenger, N., O’Connor, R., Farahmand, F., & Dehghani-Sanij, A. (2024). The Impact of Different Self-Selected Walking Speeds on Muscle Synergies in Transfemoral Amputees during Transient-State Gait. Biomechanics, 4(1), 14-33. https://doi.org/10.3390/biomechanics4010002





