Development of Bilayer Polysaccharide-Based Films Combining Extrusion and Electrospinning for Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TPS/MCC Layer
2.3. Preparation of Solutions for Electrospinning
2.4. Electrospinning
2.5. Characterization of Materials
2.5.1. Morphological Characterization
2.5.2. Structural Characterization by Fourier Transform Infrared Spectroscopy (FTIR)
2.5.3. Thermal Stability Characterization
2.5.4. Wettability
2.5.5. Oxygen Permeability
2.5.6. Mechanical Properties
2.5.7. Characterization of Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
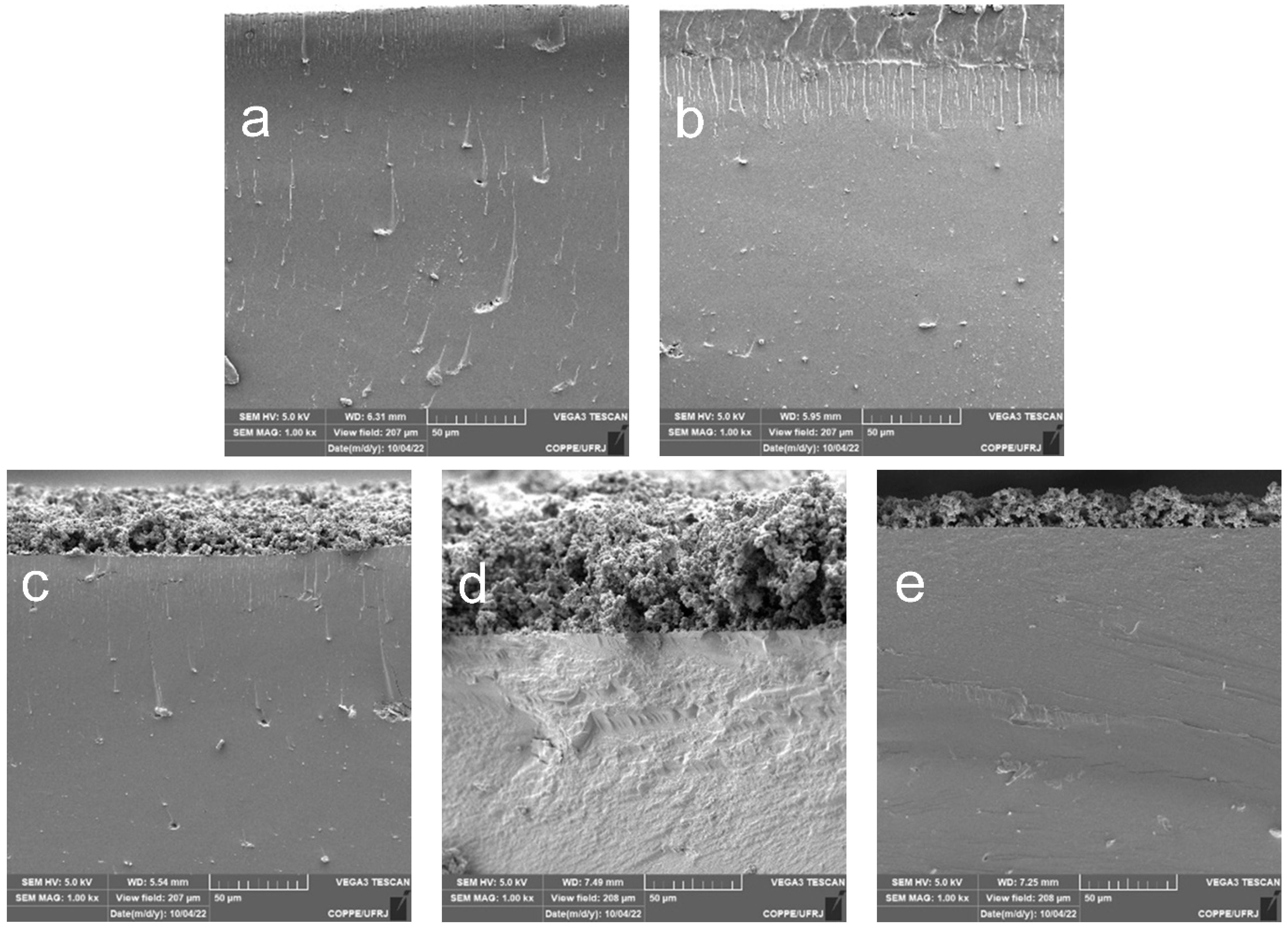
3.1. Morphological Characterization
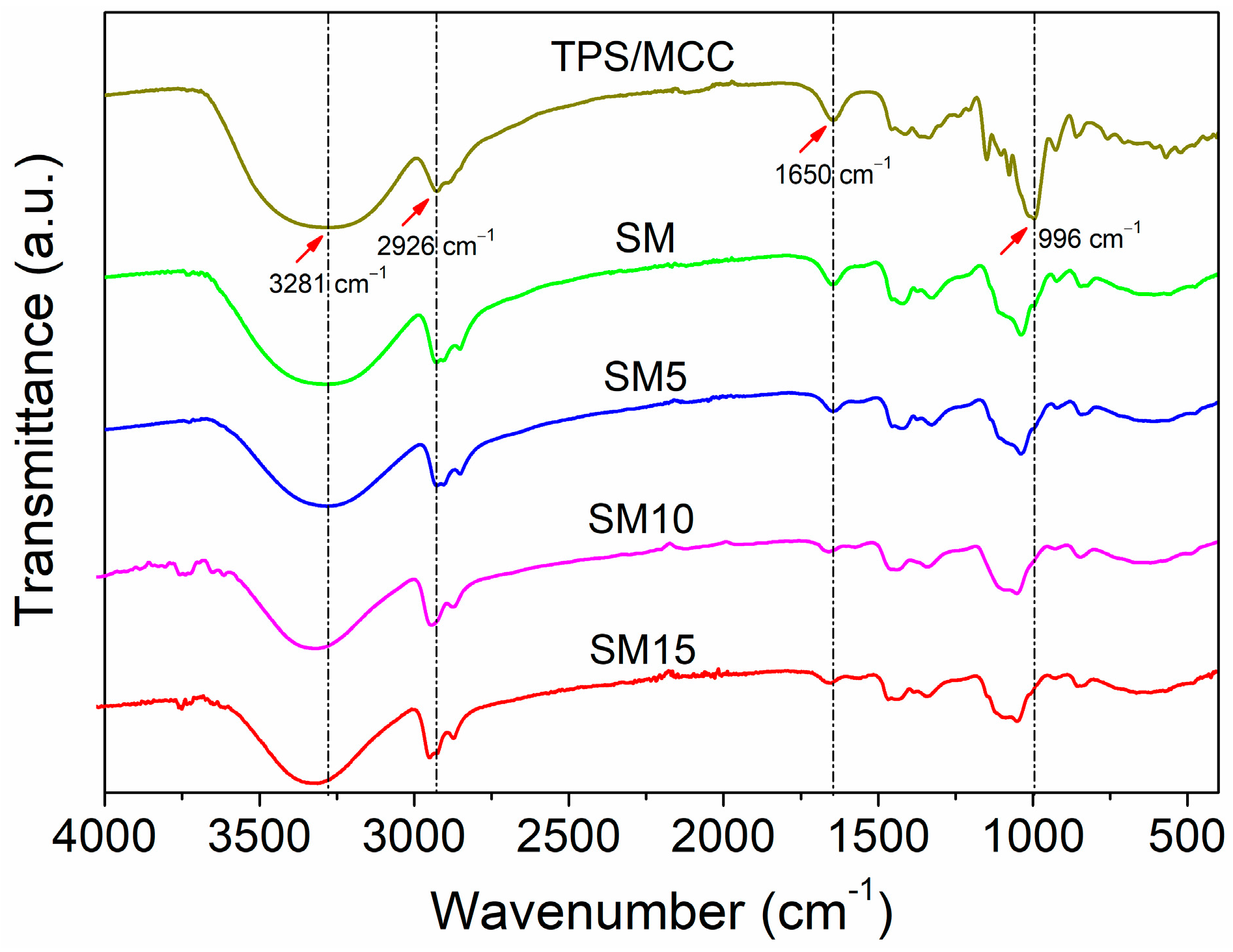
3.2. Structural Characterization by FTIR
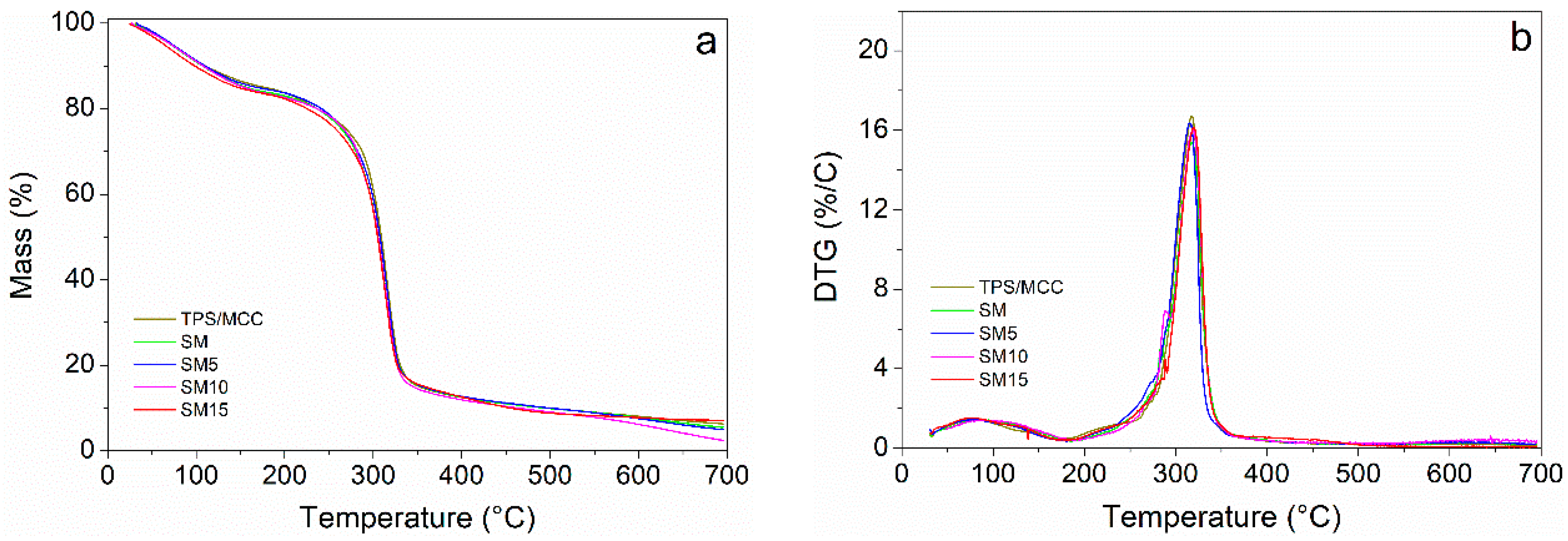
3.3. Thermal Stability Characterization
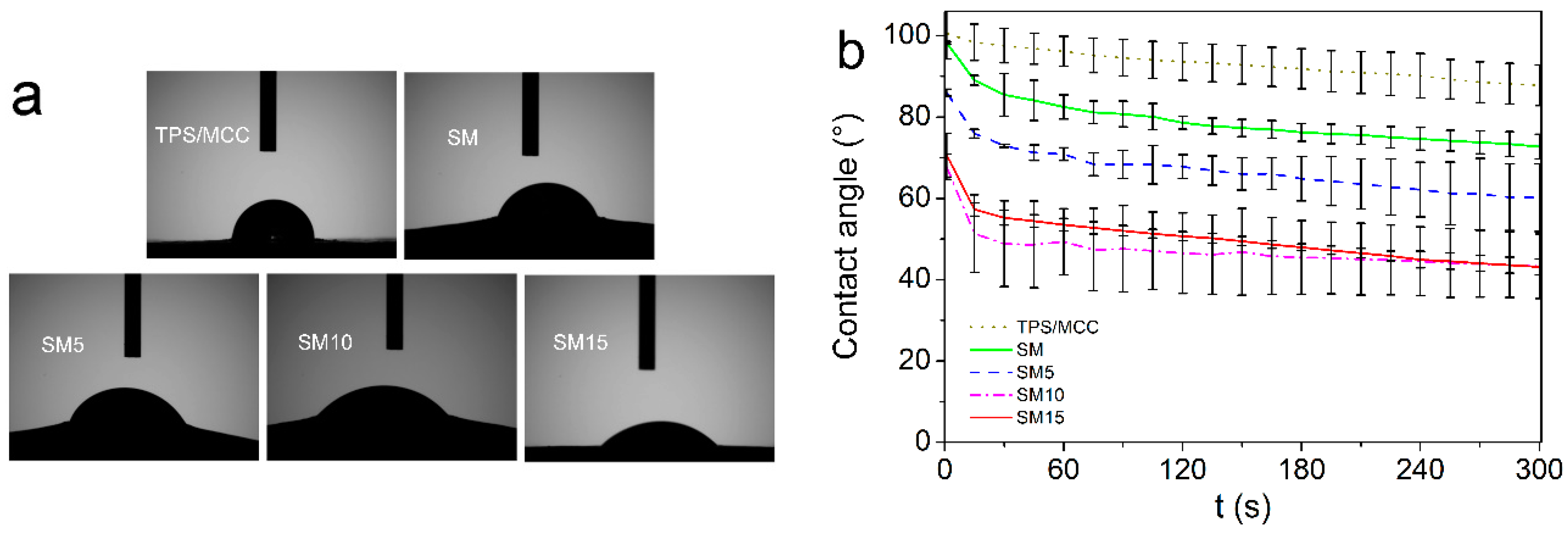
3.4. Wettability
3.5. Oxygen Permeability
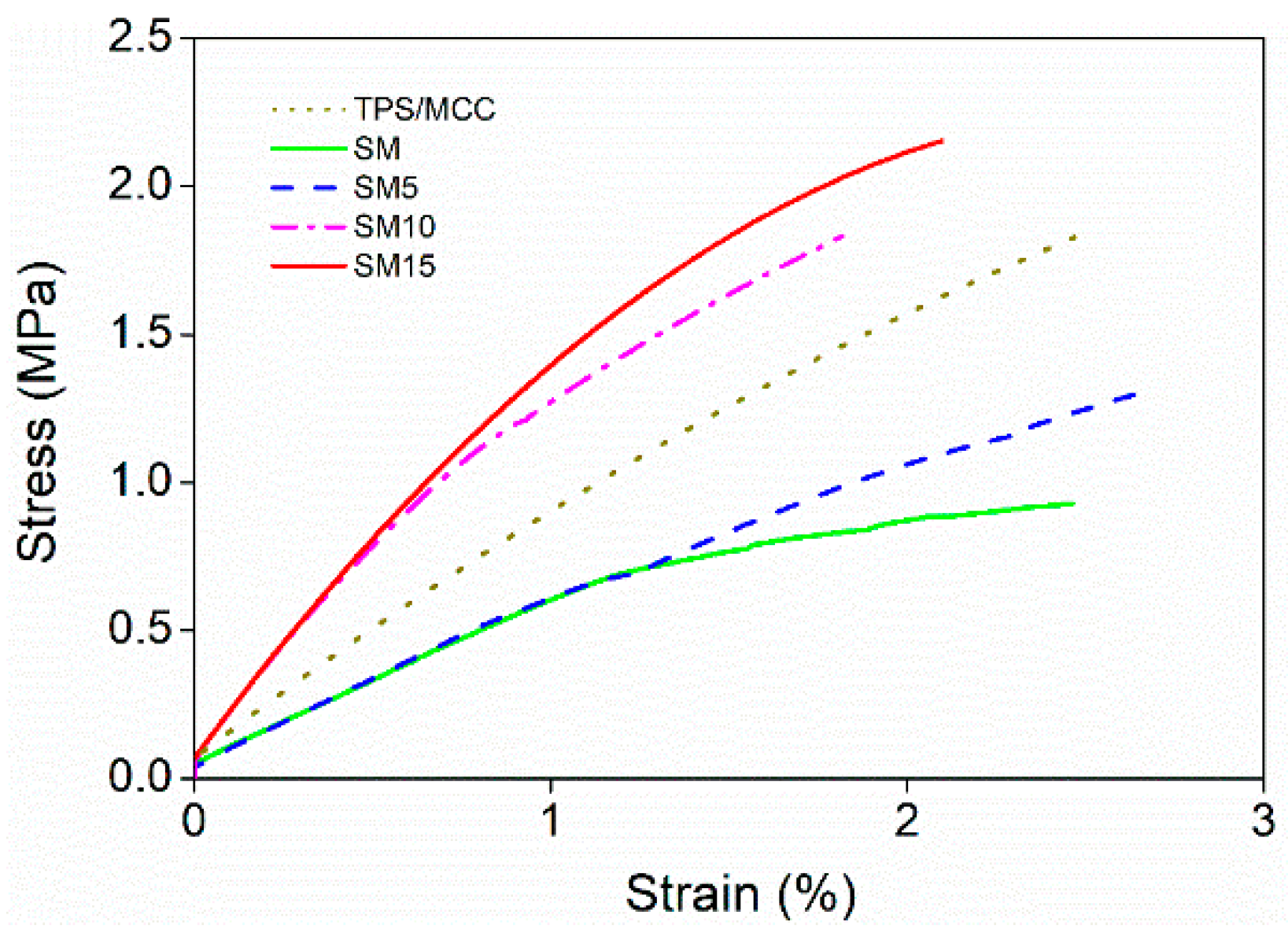
3.6. Mechanical Properties
3.7. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Numa, I.A.N.; Wolf, K.E.; Pastore, G.M. FoodTech startups: Technological solutions to achieve SDGs. Food Humanit. 2023, 1, 358–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Zhang, W.; Zhang, L.; Zhao, H.; Wang, S.; Huang, C. Recent advances in sustainable antimicrobial food packaging: Insights into release mechanisms, design strategies, and applications in the food industry. J. Agric. Food Chem. 2023, 71, 11806–11833. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F.; Wong, W.-T. Design and practical considerations for active polymeric films in food packaging. Int. J. Mol. Sci. 2022, 23, 6295. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit juice industry wastes as a source of bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, T.; Zhao, Y.; Cheng, C.; Yin, H.; Yue, J. The developments and trends of electrospinning active food packaging: A review and bibliometrics analysis. Food Control 2024, 160, 110291. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Polysaccharide-based biodegradable films: An alternative in food packaging. Polysaccharides 2022, 3, 761–775. [Google Scholar] [CrossRef]
- Popyrina, T.N.; Demina, T.S.; Akopova, T.A. Polysaccharide-based films: From packaging materials to functional food. J. Food Sci. Technol. 2022, 60, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Yang, X.; Deshmukh, R.K.; Gaikwad, K.K.; Bahmid, N.A.; Castro-Muñoz, R. Recent advances in reinforced bioplastics for food packaging—A critical review. Int. J. Biol. Macromol. 2024, 263, 130399. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. NPJ Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Biswas, D.; Roy, S.; Jamróz, E. Advances in biopolymer-based multi-layer film preparations and food packaging applications. Food Packag. Shelf Life 2023, 35, 101033. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Oliveira, L.M.; Bócoli, P.F.J.; Cristianini, M.; Padula, M.; Anjos, C.A.R. Morphological, thermal and mechanical properties of polyamide and ethylene vinyl alcohol multilayer flexible packaging after high-pressure processing. J. Food Eng. 2020, 276, 109913. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Ferreira, W.H.; Souto, L.F.C.; Gonçalves, R.P.; Soares, B.G.; Andrade, C.T. Flexible dielectric ZnO-doped reduced graphene oxide bionanocomposites from solution blending for potential application in bio-related devices. J. Appl. Polym. Sci. 2021, 138, e51186. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep Glucomannan. Carbohyd. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Chen, J.; He, J. Effects of cinnamon essential oil on the physical, mechanical, structural and thermal properties of cassava starch-based edible films. Int. J. Biol. Macromol. 2021, 184, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, G.; Yang, X.; Fan, F. Preparation and characterization of nano-SiO2-modified emulsified film and its application for strawberry preservation. Food Packag. Shelf Life 2023, 40, 101181. [Google Scholar] [CrossRef]
- ISO 527-3:2018; Plastics—Determination of Tensile Properties—Part 3 Test Conditions for Films and Sheets. International Organization for Standardization, ISO Central Secretariat: Geneva, Switzerland, 2018.
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT-Food Sci. Technol. 2020, 130, 109573. [Google Scholar] [CrossRef]
- Zarandona, I.; Puertas, A.I.; Dueñas, M.T.; Guerrero, P.; dela Caba, K. Assessment of active films incorporated with gallic acid. Food Hydrocoll. 2020, 101, 105486. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Liu, J.; Wang, P.; Jiang, S.; Li, X.; Jiang, S. Montmorillonite@chitosan-poly (ethylene oxide) nanofibrous membrane enhancing poly (vinyl alcohol-co-ethylene) composite film. Carbohydr. Polym. 2018, 181, 885–892. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Panda, P.K.; Park, K.; Seo, J. Development of poly(vinylalcohol)/regenerated chitosan blend film with superior barrier, antioxidant, and antibacterial properties. Progr. Org. Coat. 2023, 183, 107749. [Google Scholar] [CrossRef]
- Zakani, B.; Grecov, D. Effect of ultrasonic treatment on yield stress of highly concentrated cellulose nano-crystalline (CNC) aqueous suspensions. Carbohydr. Polym. 2022, 291, 119651. [Google Scholar] [CrossRef] [PubMed]
- Günzler, H.; Böck, H. IR-Spektroskopie Eine Einführung, 1st ed.; Verlarg Chemie: Weinheim, Germany, 1975; pp. 206–211. [Google Scholar]
- Li, T.; Liu, R.; Zhang, C.; Meng, F.; Wang, L. Developing a green film from locust bean gum/carboxycellulose nanocrystal for fruit preservation. Future Foods 2021, 4, 100072. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Yue, H.; Du, Z.; Cheng, X.; Wang, H.; Cheng, F.; Du, X. Flame-retardant phytic acid–decorated thermoplastic starch/halloysite nanotube composite films with enhanced mechanical strength and excellent barrier properties. Carbohyd. Polym. 2024, 323, 121465. [Google Scholar] [CrossRef]
- Ferreira, W.H.; Carmo, M.M.I.B.; Silva, A.L.N.; Andrade, C.T. Effect of structure and viscosity of the components on some properties of starch-rich hybrid blends. Carbohyd. Polym. 2015, 117, 988–995. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased ternary films of thermoplastic starch, bacterial nanocellulose and gallic acid for active food packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.-X.; He, Q.; Yu, Z.-L.; Zeng, W.-C. Interaction and action mechanism of starch with different phenolic compounds. Int. J. Food Nutr. 2020, 71, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Law, K.-L. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energ. Sources Part A 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Magalhães, N.F.; Andrade, C.T. Thermoplastic corn starch/clay hybrids: Effect of clay type and content on physical Properties. Carbohyd. Polym. 2009, 75, 712–718. [Google Scholar] [CrossRef]
- Debnath, B.; Duarah, P.; Haldar, D.; Purkait, M.K. Improving the properties of corn starch films for application as packaging material via reinforcement with microcrystalline cellulose synthesized from elephant grass. Food Packag. Shelf Life 2022, 34, 100937. [Google Scholar] [CrossRef]
- Jamróz, E.; Tkaczewska, J.; Juszczak, L.; Zimowska, M.; Kawecka, A.; Krzysciak, P.; Skóra, M. The influence of lingonberry extract on the properties of novel, double-layered biopolymer films based on furcellaran, CMC and a gelatin hydrolysate. Food Hydrocoll. 2022, 124, 107334. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-Atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Liu, Z.-Q. Chemical methods to evaluate antioxidant ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Lei, X.; Chen, Y.; Ling, J.; Zhao, J.; Ming, J. Facile fabrication of robust bilayer film loaded with chitosan active microspheres for potential multifunctional food packing. Int. J. Biol. Macromol. 2023, 231, 123362. [Google Scholar] [CrossRef] [PubMed]


| Sample A | PV * (meq/kg) | PV * (g/100 g) |
|---|---|---|
| Control | 71.2 ± 1.4 a | 0.9 ± 0.02 a |
| TPS/MCC | 66.6 ± 0.9 b | 0.85 ± 0.01 b |
| SM | 61.4 ± 1.1 c | 0.78 ± 0.01 c |
| SM5 | 60.5 ± 0.8 c | 0.76 ± 0.01 c |
| SM10 | 62.8 ± 0.9 c | 0.79 ± 0.01 c |
| SM15 | 61.1 ± 1.5 c | 0.76 ± 0.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouvêa, R.F.; Andrade, C.T. Development of Bilayer Polysaccharide-Based Films Combining Extrusion and Electrospinning for Active Food Packaging. Polysaccharides 2024, 5, 129-141. https://doi.org/10.3390/polysaccharides5020010
Gouvêa RF, Andrade CT. Development of Bilayer Polysaccharide-Based Films Combining Extrusion and Electrospinning for Active Food Packaging. Polysaccharides. 2024; 5(2):129-141. https://doi.org/10.3390/polysaccharides5020010
Chicago/Turabian StyleGouvêa, Rodrigo F., and Cristina T. Andrade. 2024. "Development of Bilayer Polysaccharide-Based Films Combining Extrusion and Electrospinning for Active Food Packaging" Polysaccharides 5, no. 2: 129-141. https://doi.org/10.3390/polysaccharides5020010