A Simple Method for Estimating Stomatal Aperture from Temperature Measurements on Intact Leaves and Wet and Dry Artificial Reference Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Outline for Determining the Stomatal Opening Index (SOI)
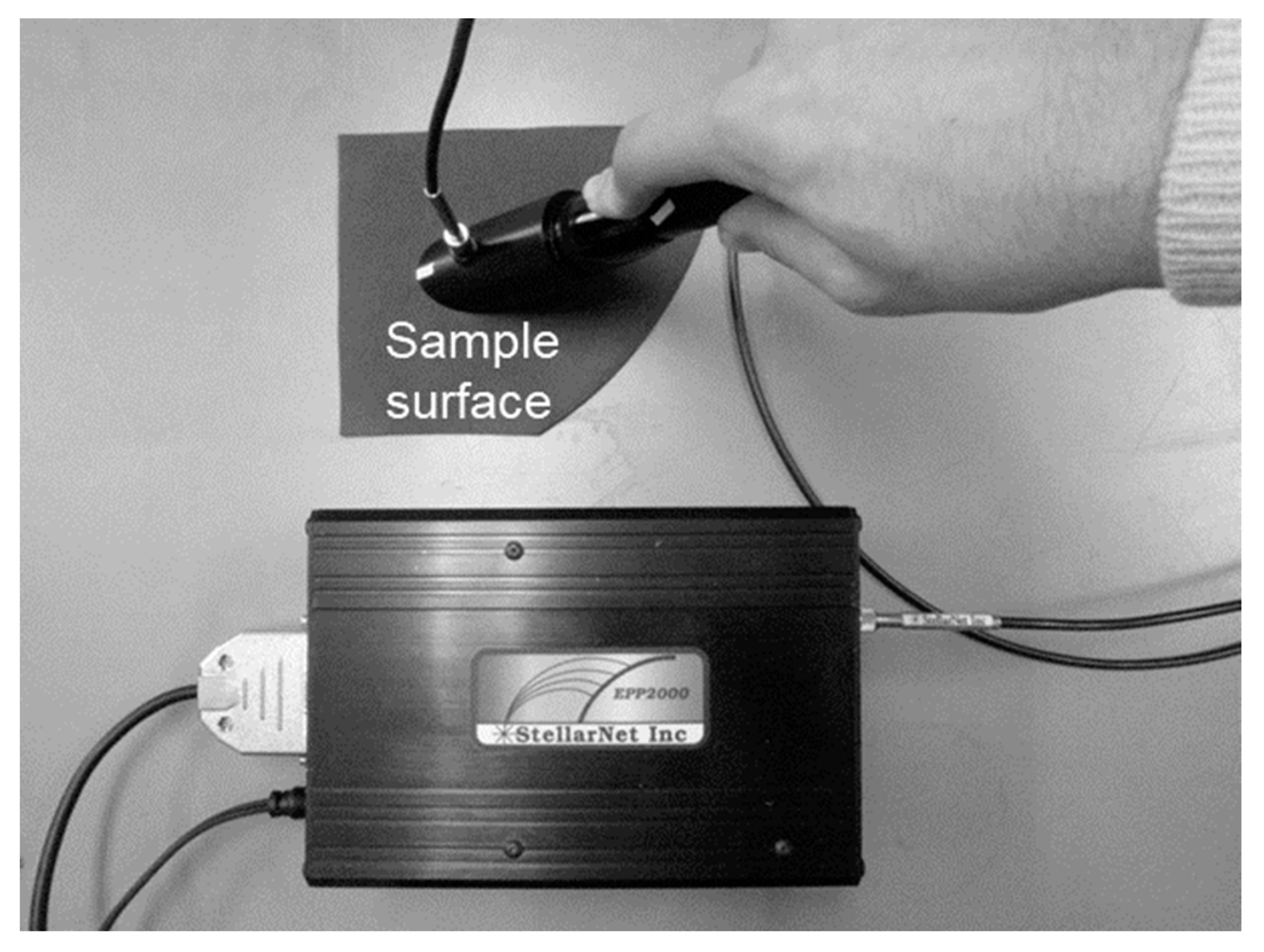
2.2. Verification of the Thermal Image Camera including Setting Values of Emissivity
2.3. Examination of Materials Used for Measurement as Reference Leaves
2.4. Radiation Reflectance, Transmittance, and Absorption of Reference and Intact Leaves
2.5. Effects of Environmental Factors on SOI Determination
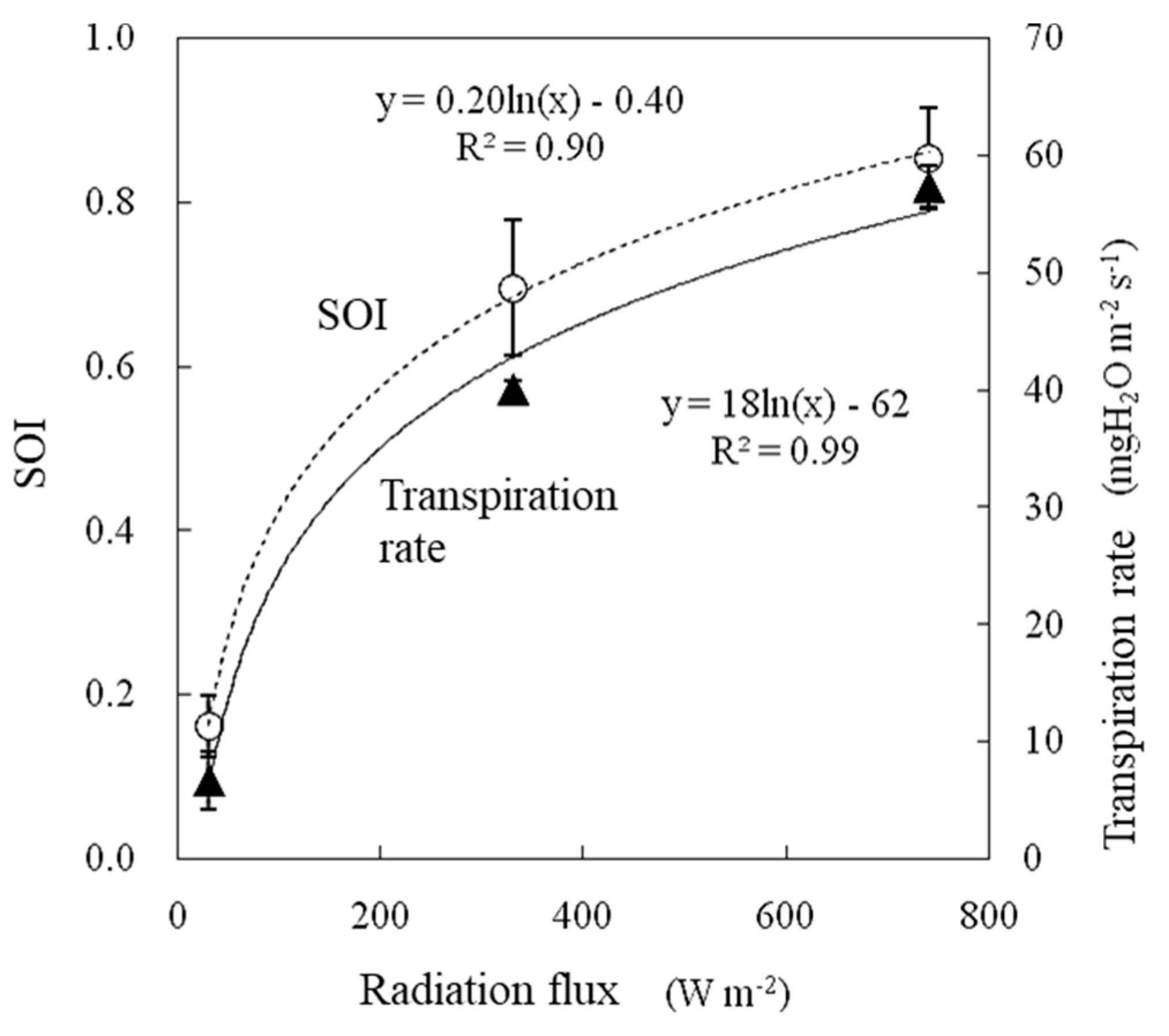
2.5.1. Radiation Flux
2.5.2. Wind Speeds
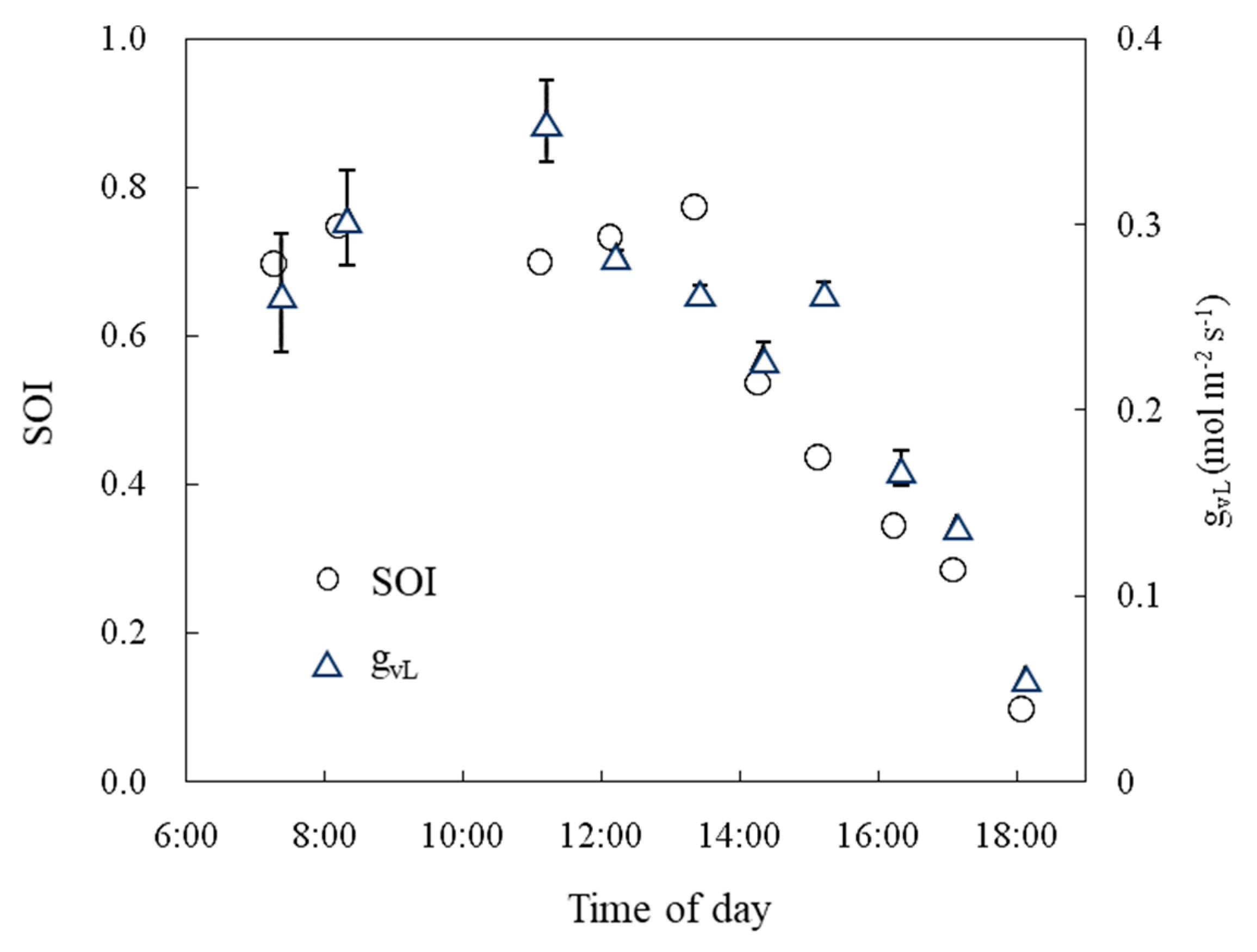
2.6. Evaluation to Confirm the Effectiveness of the SOI in a Greenhouse
3. Results and Discussion
3.1. Verification of the Thermal Image Camera including Setting Values of Emissivity
3.2. Examination of Materials Used for Measurement as Reference Leaves
3.3. Radiation Reflectance, Transmittance, and Absorption of Reference and Intact Leaves
3.4. Effects of Environmental Factors on SOI Determination
3.4.1. Radiation Flux
3.4.2. Wind Speeds
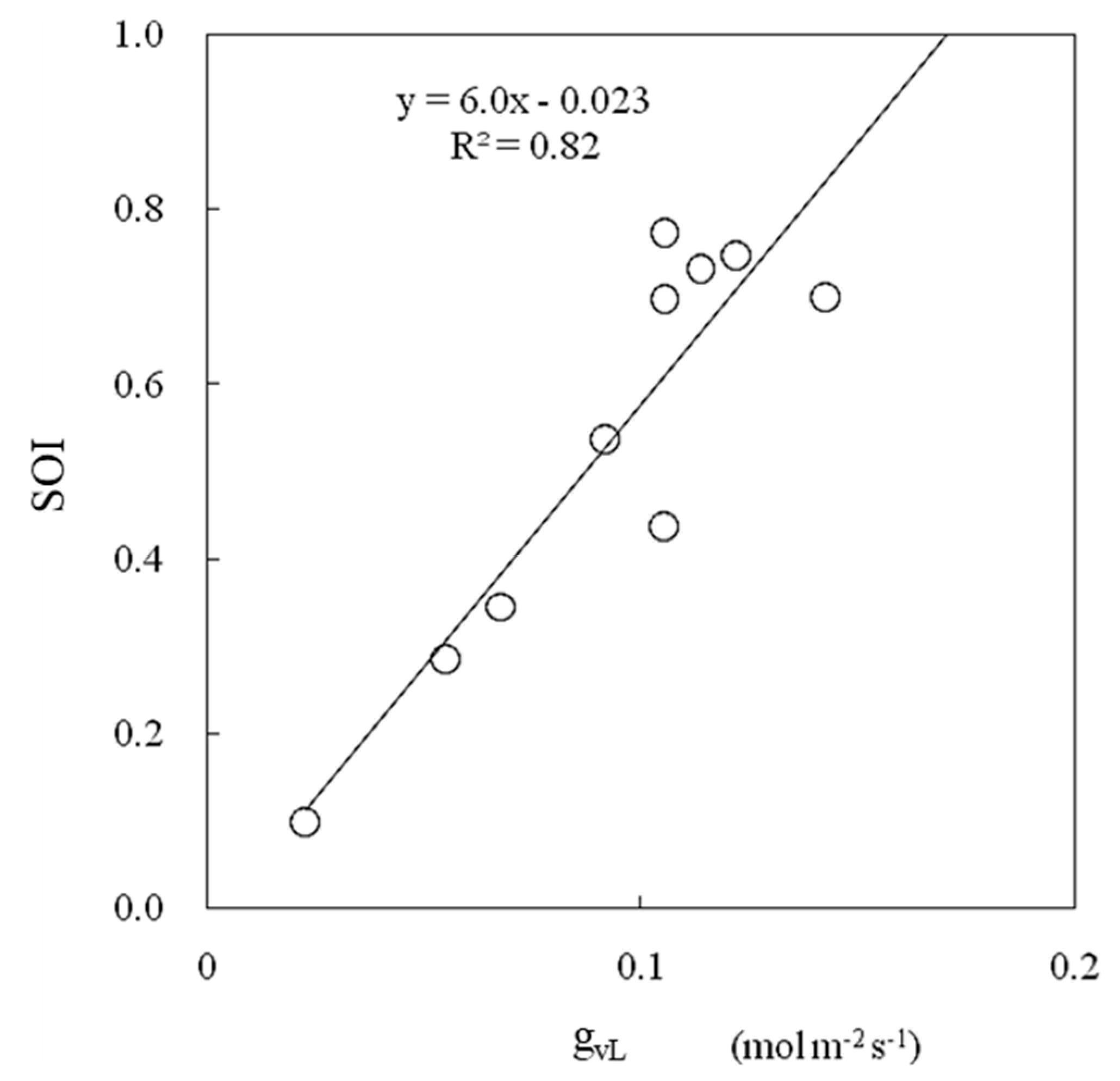
3.5. Evaluation to Confirm the Effectiveness of the SOI in a Greenhouse
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kozai, T. (Ed.) Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018; p. 441. [Google Scholar]
- Gao, Z.; Luo, Z.; Zhang, W.; Lv, Z.; Xu, Y. Deep learning application in plant stress imaging: A review. AgriEngineering 2020, 2, 29. [Google Scholar] [CrossRef]
- Zhou, Z.; Majeed, Y.; Naranjo, G.D.; Gambacorta, E.M. Assessment for crop water stress with infrared thermal imagery in precision agriculture: A review and future prospects for deep learning applications. Comput. Electron. Agric. 2020, 182, 106019. [Google Scholar] [CrossRef]
- Kozai, T.; Kubota, C.; Takagaki, M.; Maruo, T. Greenhouse environment control technologies for improving the sustainability of food production. Acta Hortic. 2014, 1107, 1–14. [Google Scholar] [CrossRef]
- Kitić, G.; Tagarakis, A.; Cselyuszka, N.; Panić, M.; Birgermajer, S.; Sakulski, D.; Matović, J. A new low-cost portable multispectral optical device for precise plant status assessment. Comput. Electron. Agric. 2019, 162, 300–308. [Google Scholar] [CrossRef]
- Achour, Y.; Ouammi, A.; Zejli, D. Technological progresses in modern sustainable greenhouses cultivation as the path toward precision agriculture. Renew. Sustain. Energy Rev. 2021, 147, 111251. [Google Scholar] [CrossRef]
- Pekkerieta, E.J.; Van Henten, E.J.; Campen, J.B. Contribution of innovative technologies to new developments in horticulture. Acta Hortic. 2015, 1099, 45–54. [Google Scholar] [CrossRef]
- Carrasco-Benavides, M.; Espinoza-Meza, S.; Umemura, K.; Ortega-Farías, S.; Baffico-Hernández, A.; Neira-Román, J.; Ávila-Sánchez, S.; Fuentes, S. Evaluation of thermal-based physiological indicators for determining water-stress thresholds in drip-irrigated ‘Regina’cherry trees. Irrig. Sci. 2024, 42, 1–15. [Google Scholar] [CrossRef]
- Poirier-Pocovi, M.; Bailey, B.N. Sensitivity analysis of four crop water stress indices to ambient environmental conditions and stomatal conductance. Sci. Hortic. 2020, 259, 108825. [Google Scholar] [CrossRef]
- Savvides, A.M.; Velez-Ramirez, A.I.; Fotopoulos, V. Challenging the water stress index concept: Thermographic assessment of Arabidopsis transpiration. Physiol. Plant. 2022, 174, e13762. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Sahoo, R.N.; Singh, P.; Patra, H.; Bajpai, V.; Das, B.; Kumar, S.; Dhandapani, R.; Vishwakarma, C.; Pal, M. Application of thermal imaging and hyperspectral remote sensing for crop water deficit stress monitoring. Geocarto Int. 2021, 36, 481–498. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Lawson, T. Dynamic leaf energy balance: Deriving stomatal conductance from thermal imaging in a dynamic environment. J. Exp. Bot. 2019, 70, 2839–2855. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Lawson, T. Thermography methods to assess stomatal behavior in a dynamic environment. J. Exp. Bot. 2020, 71, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Monteith, J.L. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 1995, 18, 357–364. [Google Scholar] [CrossRef]
- Guilioni, L.; Leinonen, I.; Lhomme, J.P. On the relationships between stomatal resistance and leaf temperatures in thermography. Agric. For. Meteology 2008, 148, 1908–1912. [Google Scholar] [CrossRef]
- Jones, H.G. The use of infrared thermometry for estimation of stomatal conductance is a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H.G. Estimating stomatal conductance with thermal imagery. Plant Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Struthers, R.; Ivanova, A.; Tits, L.; Swennen, R.; Coppin, P. Thermal infrared imaging of the temporal variability in stomatal conductance for fruit trees. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 9–17. [Google Scholar] [CrossRef]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.L. Thermal imaging for plant stress detection and phenotyping. Remote Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Yabuki, K. Photosynthetic Rate and Dynamic Environment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; p. 136. [Google Scholar]
- Kitaya, Y.; Tsuruyama, J.; Shibuya, T.; Yoshida, M.; Kiyota, M. Effects of air current speed on gas exchange in plant leaves and plant canopies. Adv. Space Res. 2003, 31, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Suwannarut, W.; Vialet-Chabrand, S.; Kaiser, E. Diurnal decline in photosynthesis and stomatal conductance in several tropical species. Front. Plant Sci. 2023, 14, 1273802. [Google Scholar] [CrossRef] [PubMed]
- Gontia, N.K.; Tiwari, K.N. Development of crop water stress index of wheat crop for scheduling irrigation using infrared thermometry. Agric. Water Manag. 2008, 95, 1144–1152. [Google Scholar] [CrossRef]
- Maes, W.; Achten, W.M.J.; Reubens, B.; Muys, B. Monitoring stomatal conductance of Jatropha curcas seedlings under different levels of water shortage with infrared thermography. Agric. For. Meteorol. 2011, 151, 554–564. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in superhigh density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and future perspectives of multi-/hyperspectral thermal infrared remote sensing for crop water-stress detection: A review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
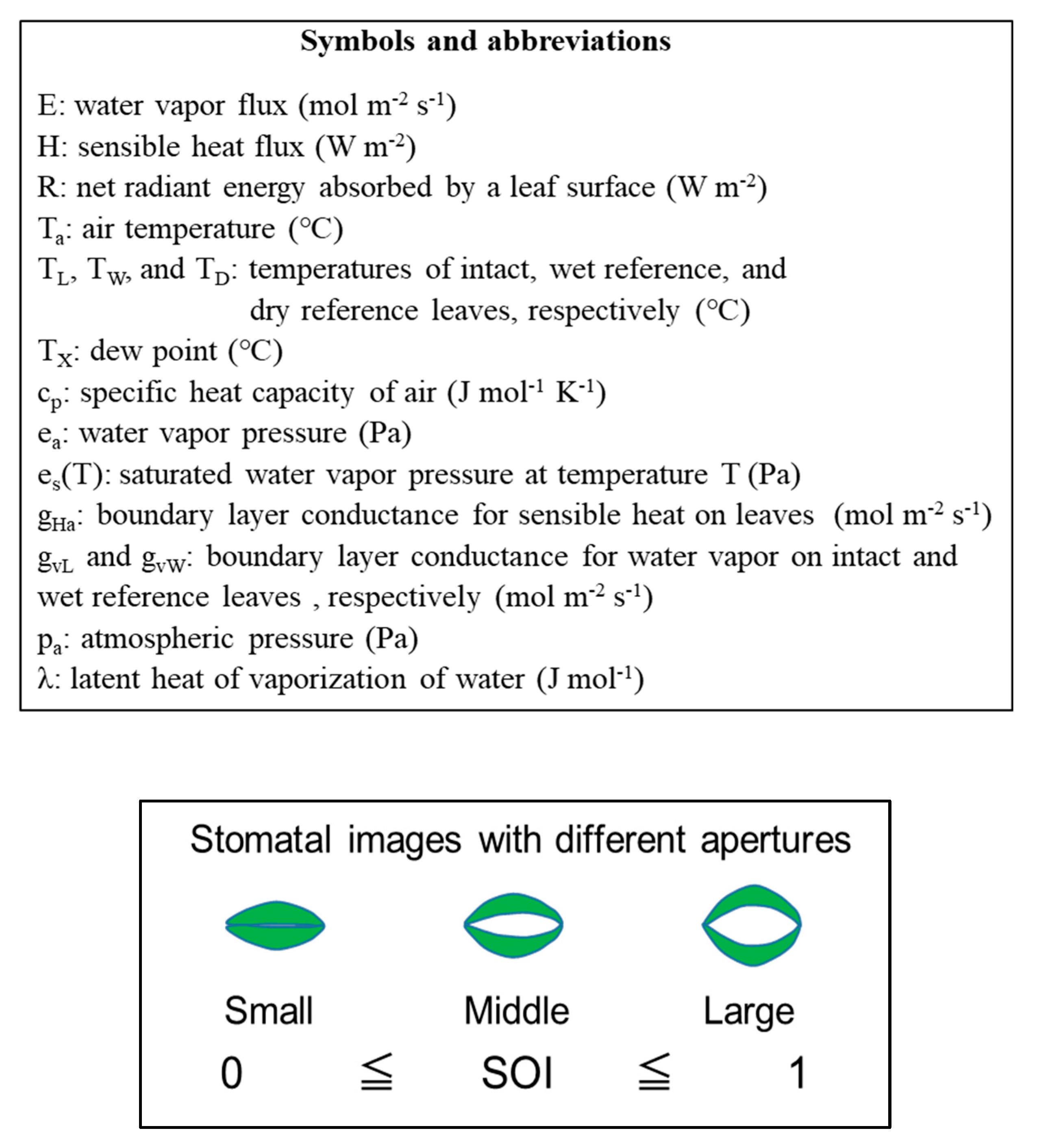










| Radiation | Intact Leaves | Dry Reference Leaves | Wet Reference Leaves |
|---|---|---|---|
| Reflectance (%) | 10.7 | 1.6 | 7.7 |
| Transmittance (%) | 27.0 | 0.0 | 23.9 |
| Absorption (%) | 62.3 | 98.4 | 68.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaya, Y.; Ikeda, N.; Endo, R.; Shibuya, T. A Simple Method for Estimating Stomatal Aperture from Temperature Measurements on Intact Leaves and Wet and Dry Artificial Reference Leaves. AgriEngineering 2024, 6, 1335-1348. https://doi.org/10.3390/agriengineering6020077
Kitaya Y, Ikeda N, Endo R, Shibuya T. A Simple Method for Estimating Stomatal Aperture from Temperature Measurements on Intact Leaves and Wet and Dry Artificial Reference Leaves. AgriEngineering. 2024; 6(2):1335-1348. https://doi.org/10.3390/agriengineering6020077
Chicago/Turabian StyleKitaya, Yoshiaki, Noboru Ikeda, Ryosuke Endo, and Toshio Shibuya. 2024. "A Simple Method for Estimating Stomatal Aperture from Temperature Measurements on Intact Leaves and Wet and Dry Artificial Reference Leaves" AgriEngineering 6, no. 2: 1335-1348. https://doi.org/10.3390/agriengineering6020077






