Electrospun Si and Si/C Fiber Anodes for Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
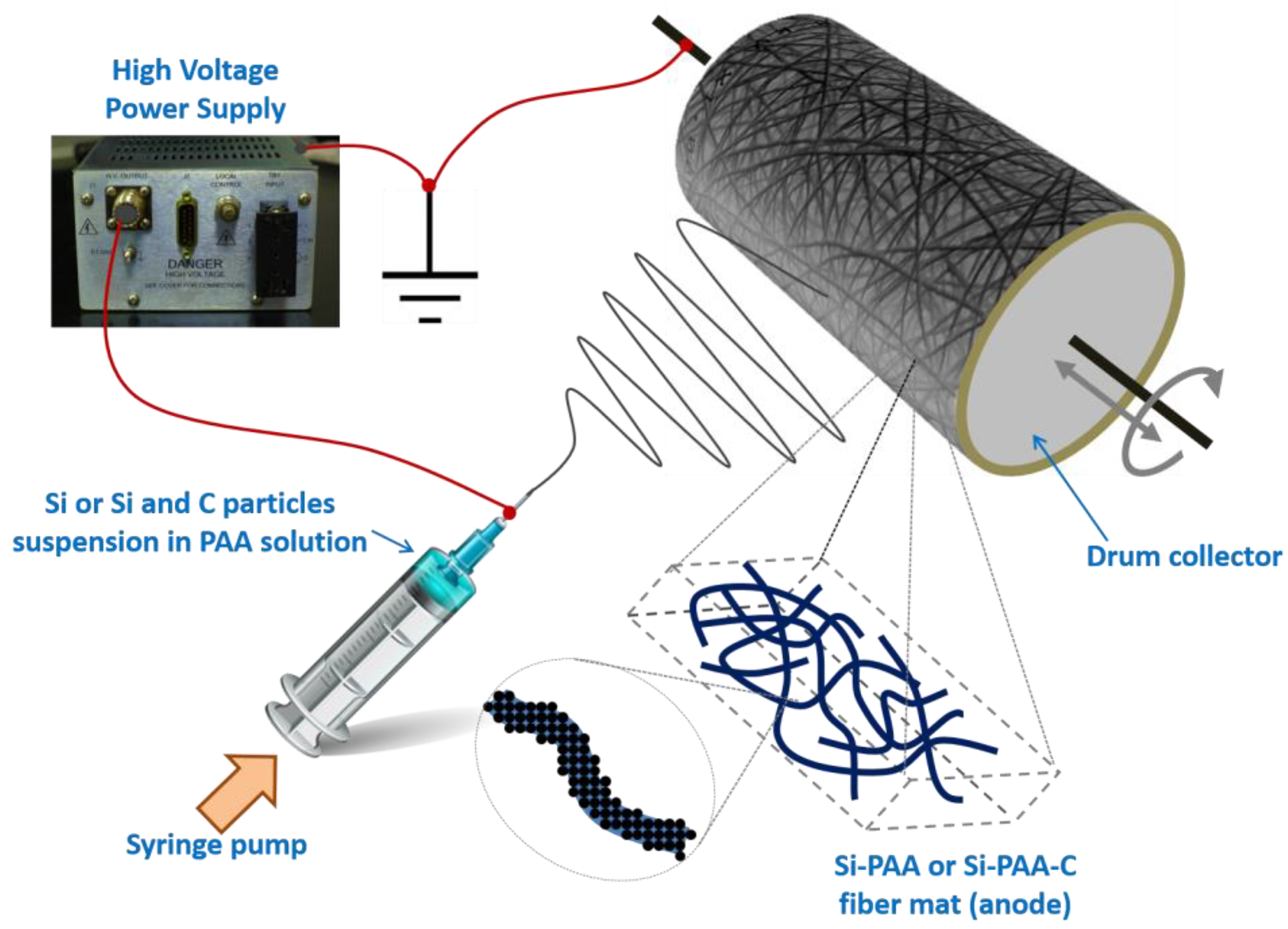
2.1. Electrospinning Si/PAA and Si/PAA/C Nanofiber Mats
2.2. Half-Cell Testing
3. Results
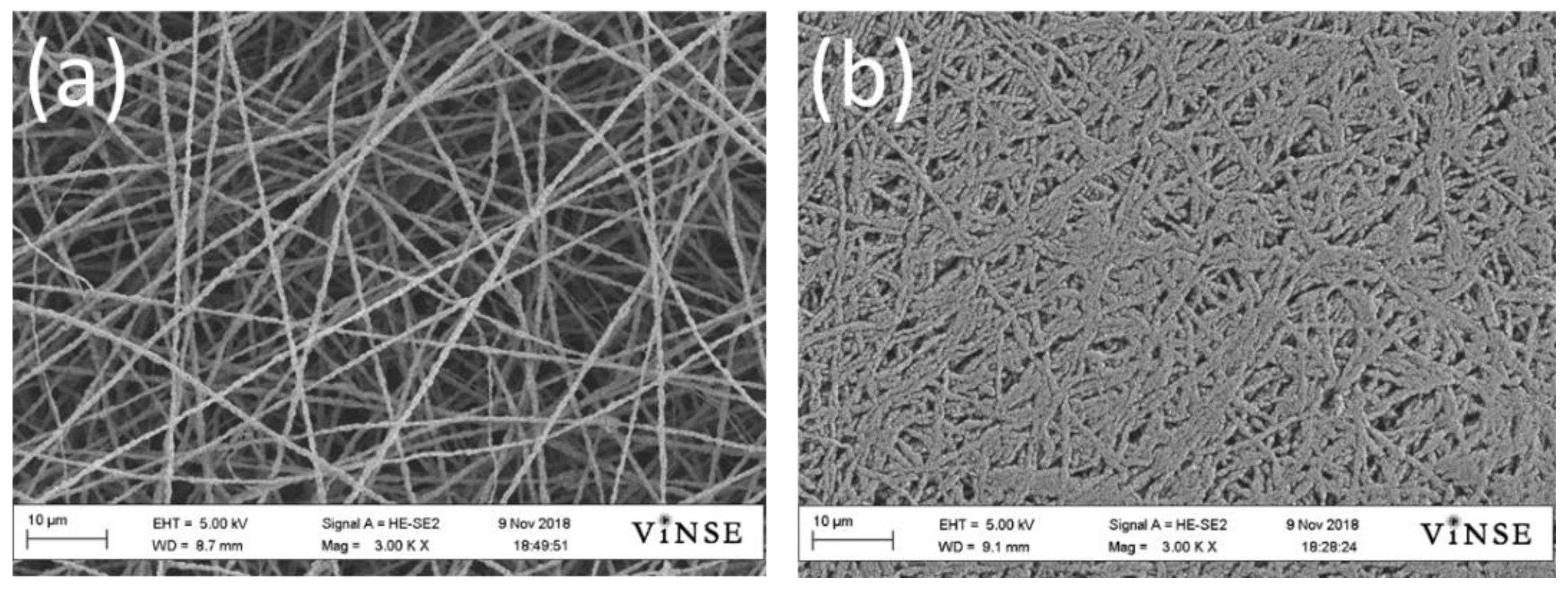
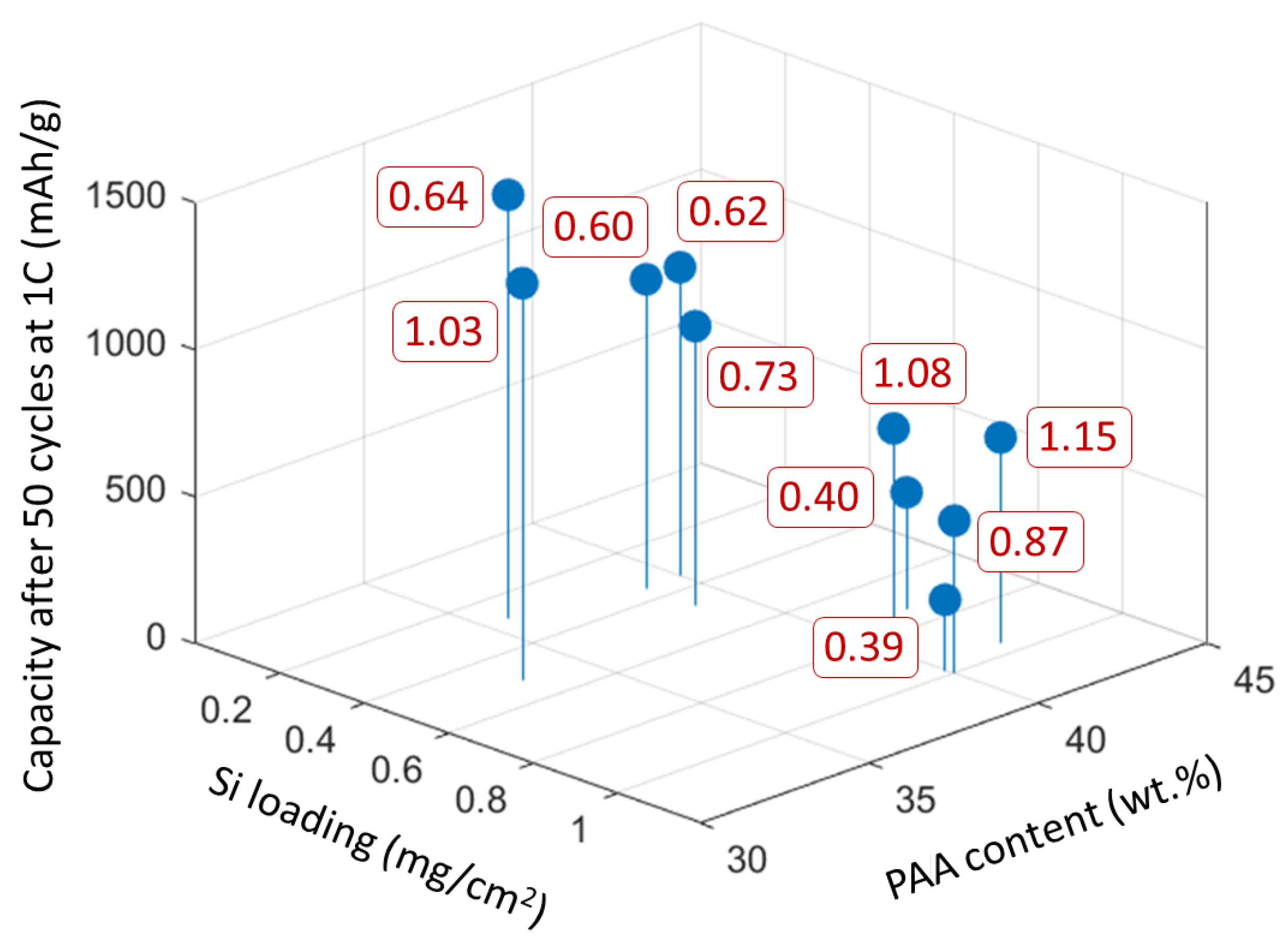
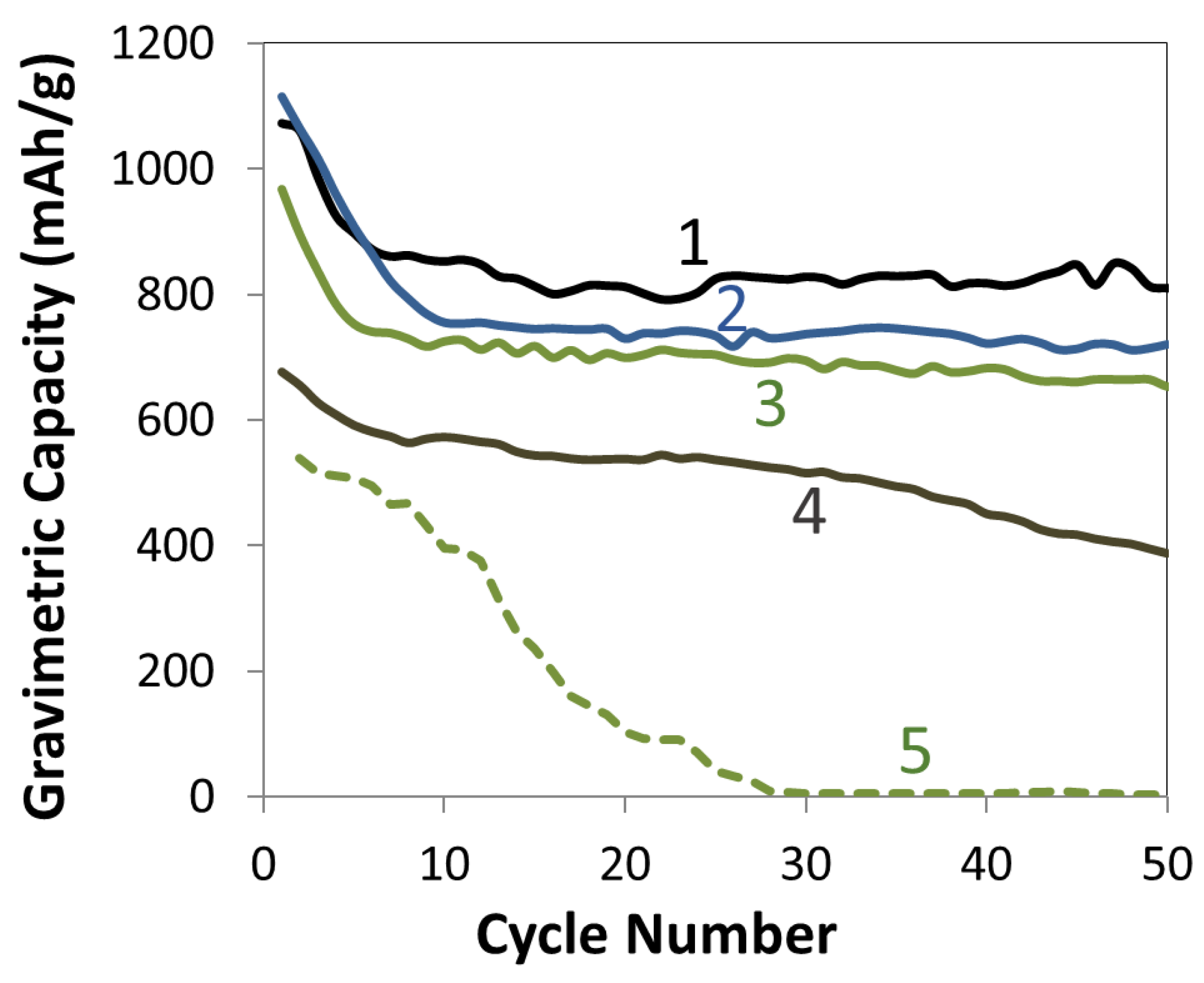
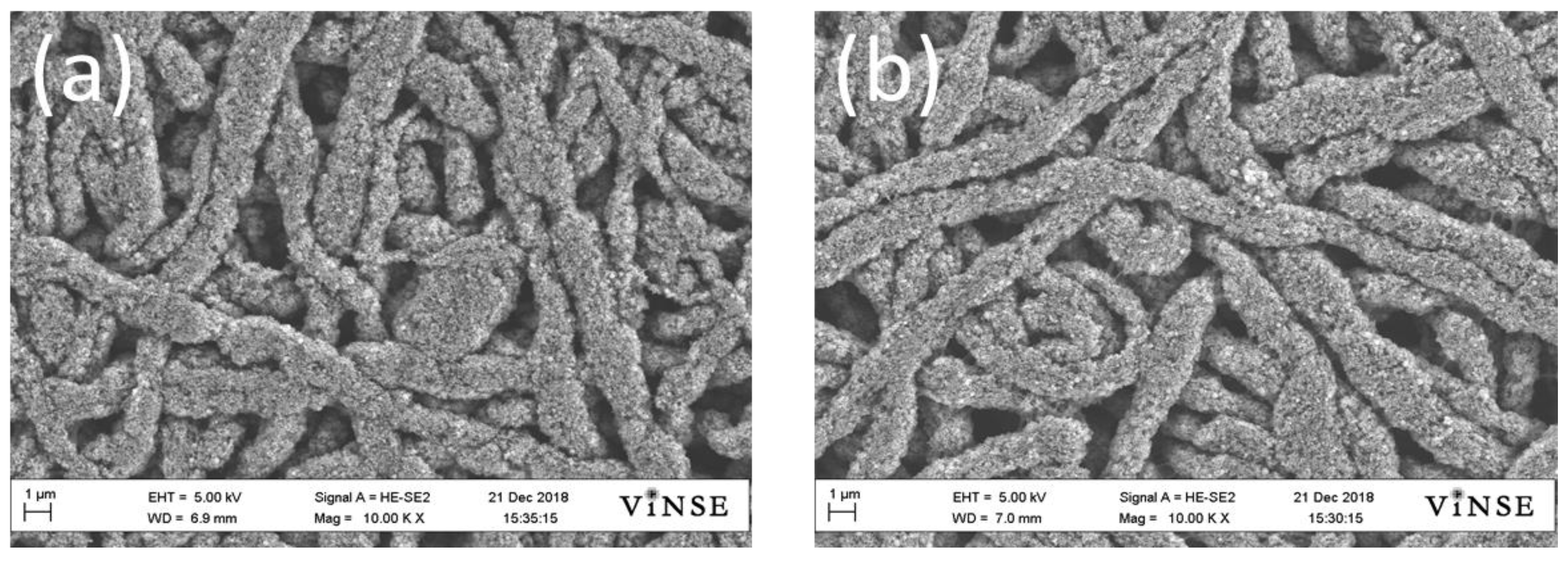
3.1. Electrospun Anodes
3.2. Electrospun Si/PAA/C Anodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical Energy Storage for Transportation-Approaching the Limits of, and Going beyond, Lithium-Ion Batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-Based Anodes for Lithium-Ion Batteries: Effectiveness of Materials Synthesis and Electrode Preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, T.; Yang, X.G.; Ge, S.; Stanley, N.V.; Rountree, E.S.; Leng, Y.; McCarthy, B.D. Fast Charging of Energy-Dense Lithium-Ion Batteries. Nature 2022, 611, 485–490. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A Non-Academic Perspective on the Future of Lithium-Based Batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- Feng, L.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Huang, G.; Han, J.; Lu, Z.; Wei, D.; Kashani, H.; Watanbe, K.; Chen, M. Ultrastable Silicon Anode by Three-Dimensional Nanoarchitecture Design. ACS Nano 2020, 14, 4374–4382. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 110. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent Progress and Future Perspective on Practical Silicon Anode-Based Lithium Ion Batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Gu, L.; Han, J.; Chen, M.; Zhou, W.; Wang, X.; Xu, M.; Lin, H.; Liu, H.; Chen, H.; Chen, J.; et al. Enabling Robust Structural and Interfacial Stability of Micron-Si Anode Toward High-Performance Liquid and Solid-State Lithium-Ion Batteries. Energy Storage Mater. 2022, 52, 547–561. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.Y.; Sun, Y.K. Nano/Microstructured Silicon-Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xiang, L.; Miao, S.; Shi, L.; Xie, D.; Yan, J.; Zheng, Z.; Zhang, X.; Tang, Y. Flexible Interface Design for Stress Regulation of a Silicon Anode toward Highly Stable Dual-Ion Batteries. Adv Mater. 2020, 32, 1908470. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, Y. Designing Nanostructured Si Anodes for High Energy Lithium Ion Batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Wang, F.; Ruan, T.; Gao, T.; Song, R.; Jin, F.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Construction of Structure-Tunable Si@Void@C Anode Materials for Lithium-Ion Batteries through Controlling the Growth Kinetics of Resin. ACS Nano 2019, 13, 12219–12229. [Google Scholar] [CrossRef]
- Li, J.Y.; Xu, Q.; Li, G.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Research Progress Regarding Si-Based Anode Materials Towards Practical Application in High Energy Density Li-Ion Batteries. Mater. Chem. Front. 2017, 1, 1691–1708. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Marquis, C.B.; et al. Progressive Growth of the Solid–Electrolyte Interphase Towards the Si Anode Interior Causes Capacity Fading. Nat. Nanotechnol. 2021, 16, 1113–1120. [Google Scholar] [CrossRef]
- Wang, M.S.; Wang, G.L.; Wang, S.; Zhang, J.; Wang, J.; Zhong, W.; Tang, F.; Yang, Z.L.; Zheng, J.; Li, X. In Situ Catalytic Growth 3D Multi-Layers Graphene Sheets Coated Nano-Silicon Anode for High Performance Lithium-Ion Batteries. J. Chem. Eng. 2019, 356, 895–903. [Google Scholar] [CrossRef]
- Jang, W.; Kim, S.; Kang, Y.; Yim, T.; Kim, T.H. A High-Performance Self Healing Polymer Binder for Si Anodes Based on Dynamic Carbon Radicals in Cross-Linked Poly(Acrylic Acid). J. Chem. Eng. 2023, 469, 143949. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Dou, Y.; Cheng, N.; Cui, D.; Du, Y.; Liu, P.; Al-Mamum, M.; Zhang, S.; Zhao, H. A Yolk-Shell Structured Silicon Anode with Superior Conductivity and High Tap Density for Full Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 8824–8828. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, C.; Cao, B.; Xu, Y.; Zhang, D.; Li, A.; Zhou, J.; Ma, Z.; Chen, X.; Song, H. One-Step Synthesis of Spherical Si/C Composites with Onion-Like Buffer Structure as High-Performance Anodes for Lithium-Ion Batteries. Energy Storage Mater. 2020, 24, 312–318. [Google Scholar] [CrossRef]
- Waldrop, K.; Slack, J.J.; Gumeci, C.; Parrondo, J.; Dale, N.; Shawn Reeves, K.; Cullen, D.A.; More, K.L.; Pintauro, P.N. Electrospun Nanofiber Electrodes for High and Low Humidity PEMFC Operation. J. Electrochem. Soc. 2023, 170, 024507. [Google Scholar] [CrossRef]
- Waldrop, K.; Wycisk, R.; Pintauro, P.N. Application of electrospinning for the fabrication of proton-exchange membrane fuel cell electrodes. Curr. Opin. Electrochem. 2020, 21, 257–265. [Google Scholar] [CrossRef]
- Self, E.C.; McRen, E.C.; Wycisk, R.; Pintauro, P.N. LiCoO2-Based Fiber Cathodes for Electrospun Full Cell Li-Ion Batteries. Electrochim. Acta 2016, 214, 139–146. [Google Scholar] [CrossRef]
- Self, E.; Naguib, M.; Ruther, R.E.; McRen, E.C.; Wycisk, R.; Liu, G.; Nanda, J.; Pintauro, P.N. High Areal Capacity Si/LiCoO2 Batteries from Electrospun Composite Fiber Mats. ChemSusChem 2017, 10, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhang, J.; Chen, J.; Du, C.; Sun, T.; Liu, J.; Gong, C.; Guo, J.; Yu, L.; et al. Sandwich Structure of Carbon-Coated Silicon/Carbon Nanofiber Anodes for Lithium-Ion Batteries. Ceram. Int. 2019, 45, 16195–16201. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhu, X.; Li, F.; Lai, F.; Wu, Y.; Miao, Y.E.; Liu, T. Silicon @ Nitrogen-Doped Porous Carbon Fiber Composite Anodes Synthesized by an In-Situ G.; Reaction Collection Strategy for High-Performance Lithium-Ion Batteries. Appl. Surf. Sci. 2019, 475, 211–218. [Google Scholar] [CrossRef]
- Park, S.J.; Zhao, H..; Ai, G.; Wang, C.; Song, X.Y.; Yuca, N.; Battaglia, V.S.; Yang, W.L.; Liu, G. Side-Chain Conducting and Phase-Separated Polymeric Binders for High-Performance Silicon Anodes in Lithium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2565–2571. [Google Scholar] [CrossRef]


| C Content (wt.%) | Total Solids Loading (mg/cm2) | Si Loading (mgSi/cm2) | Areal Capacity after 50 Cycles at 1C (mAh/cm2) |
|---|---|---|---|
| 3 | 1.7 | 0.97 | 1.38 |
| 6 | 1.3 | 0.70 | 0.94 |
| 12 | 1.3 | 0.62 | 0.85 |
| 9 | 2.3 | 1.17 | 0.89 |
| 12 | 2.9 | 1.39 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, A.N.; Wycisk, R.; Waugh, J.; Pintauro, P.N. Electrospun Si and Si/C Fiber Anodes for Li-Ion Batteries. Batteries 2023, 9, 569. https://doi.org/10.3390/batteries9120569
Mondal AN, Wycisk R, Waugh J, Pintauro PN. Electrospun Si and Si/C Fiber Anodes for Li-Ion Batteries. Batteries. 2023; 9(12):569. https://doi.org/10.3390/batteries9120569
Chicago/Turabian StyleMondal, Abhishek N., Ryszard Wycisk, John Waugh, and Peter N. Pintauro. 2023. "Electrospun Si and Si/C Fiber Anodes for Li-Ion Batteries" Batteries 9, no. 12: 569. https://doi.org/10.3390/batteries9120569







