Xyloglucan–Cellulose Nanocrystals Mixtures: A Case Study of Nanocolloidal Hydrogels and Levers for Tuning Functional Properties
Abstract
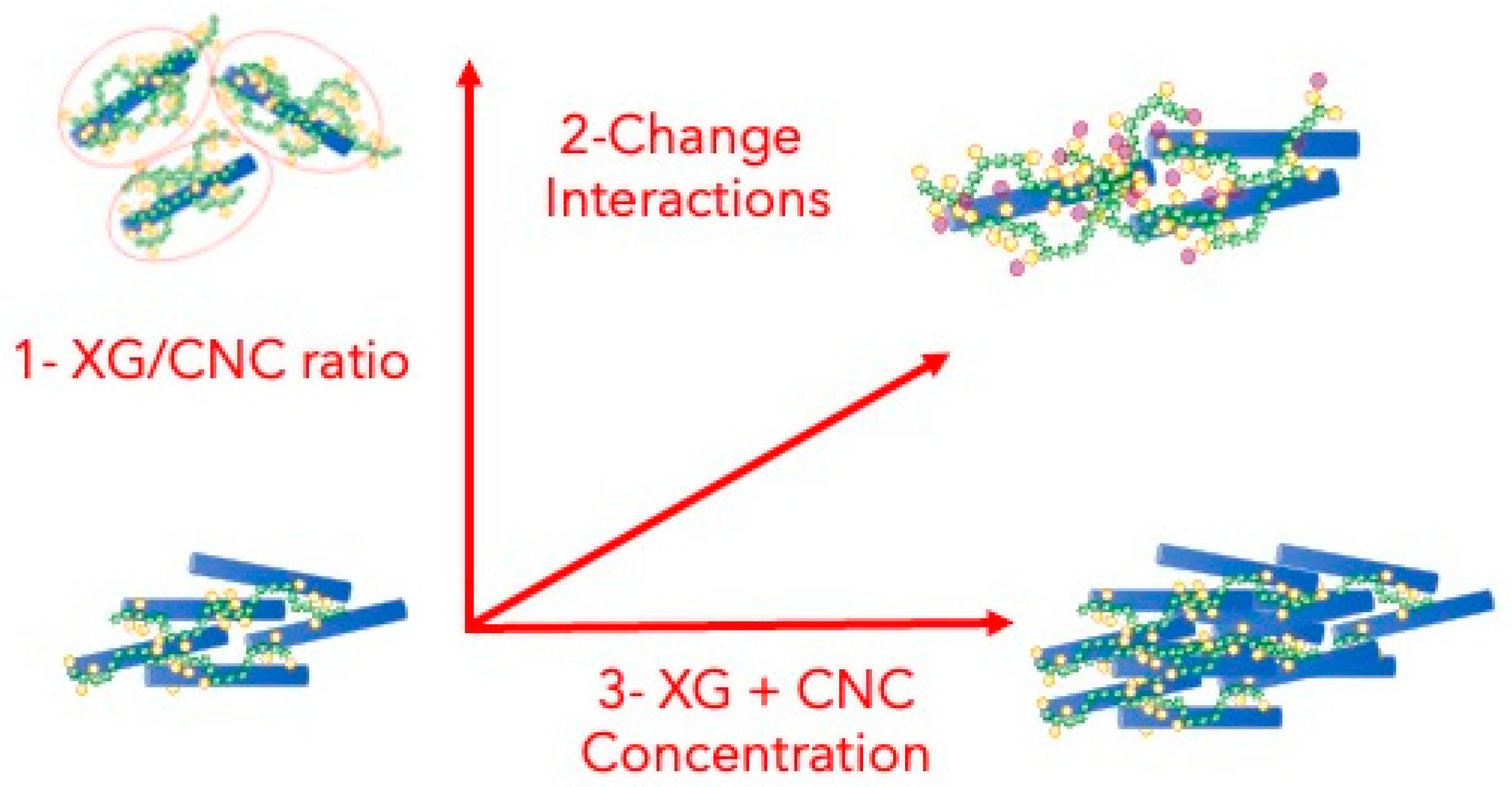
1. Introduction
2. Results and Discussion
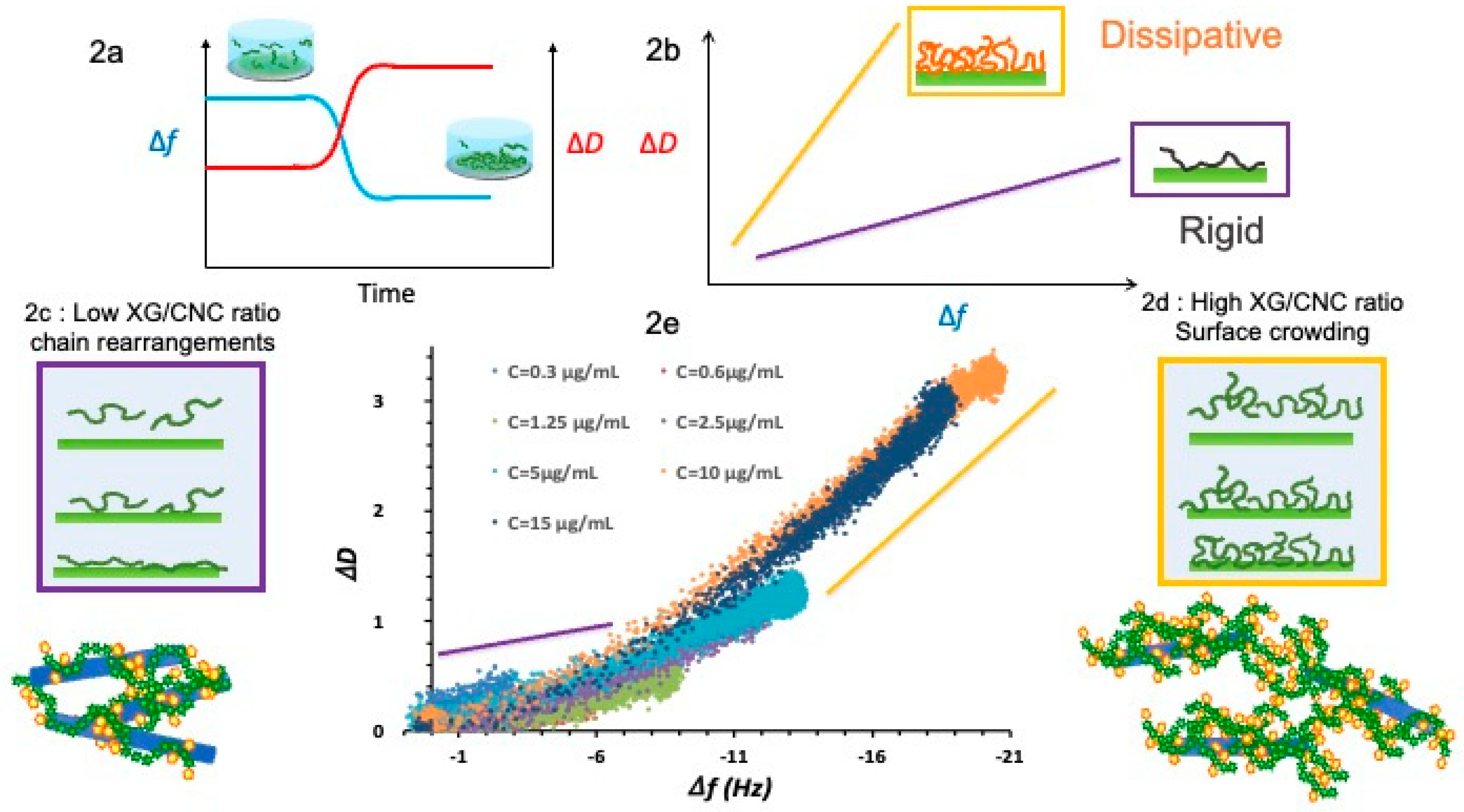
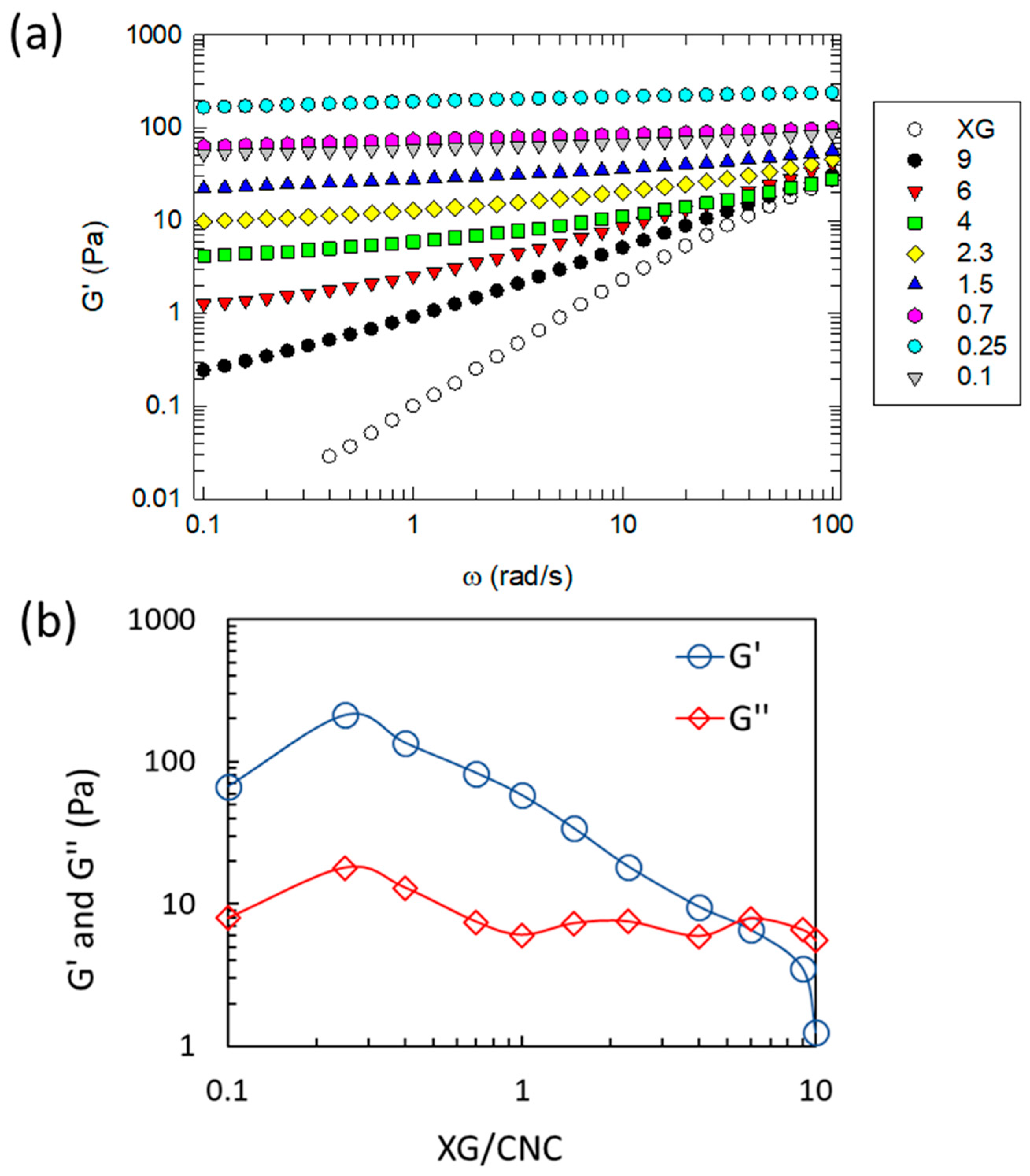
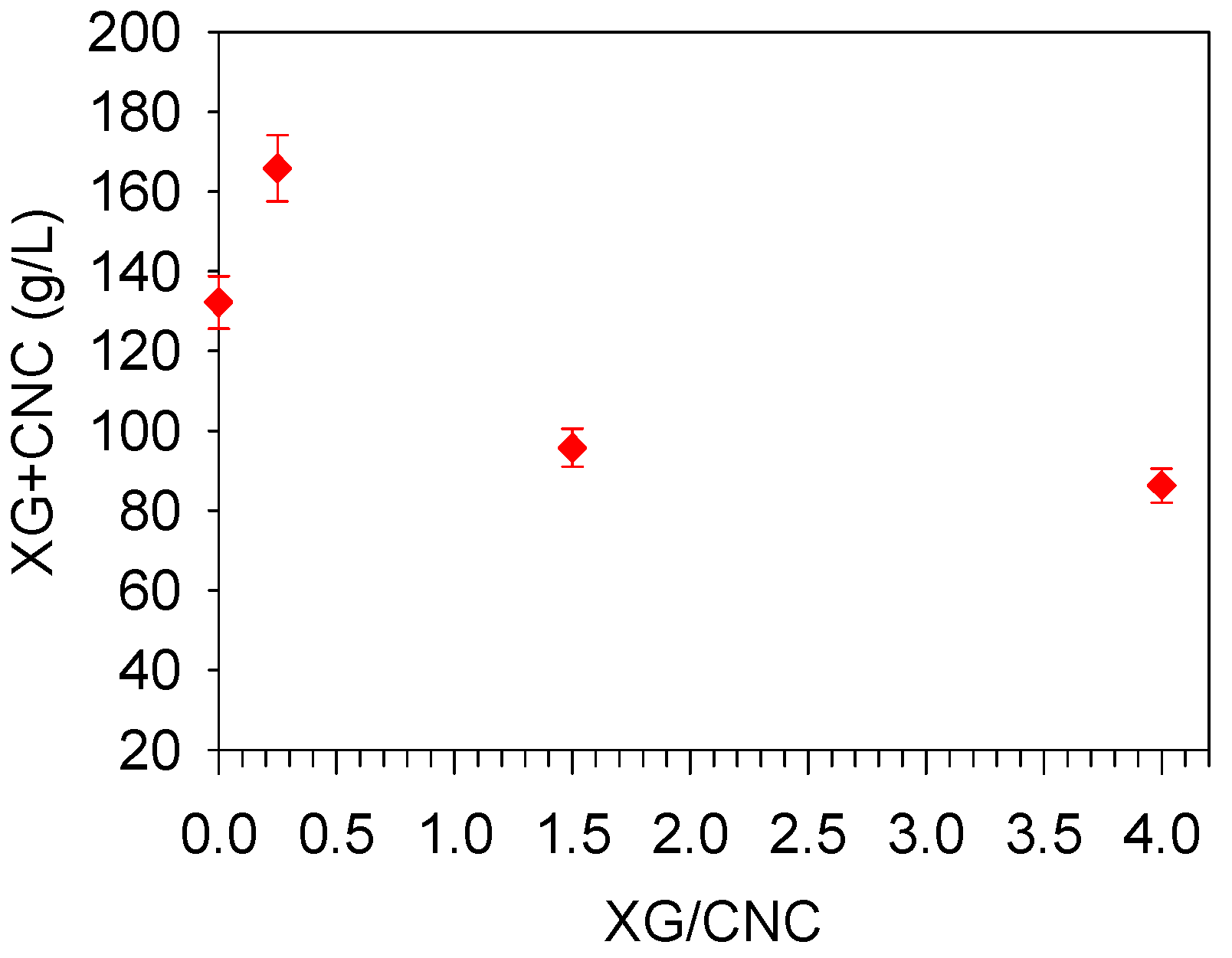
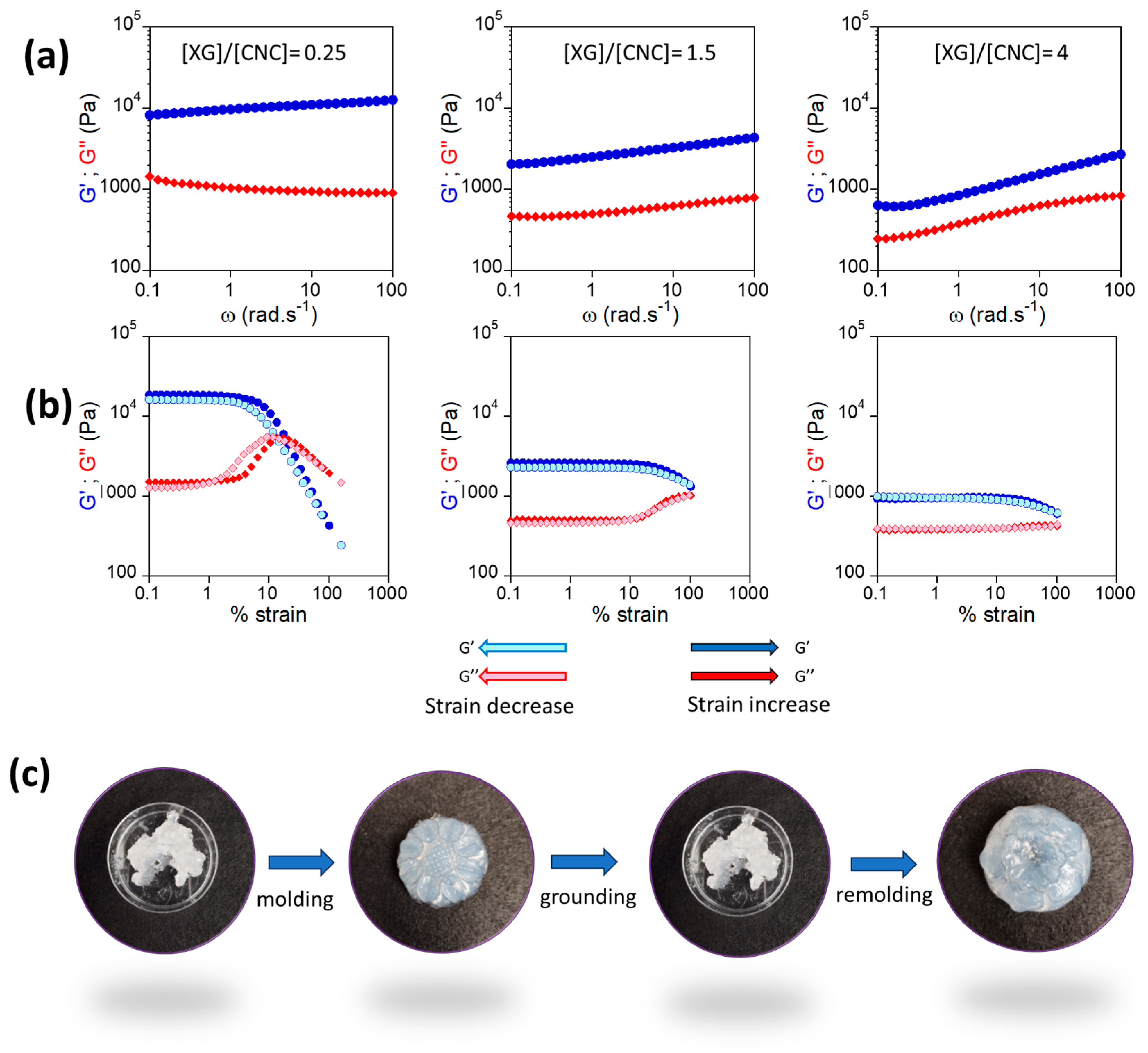
2.1. Impact of the XG/CNC Ratio
2.2. Reinforcing Xyloglucan Interactions: Toward Thermoresponsive Hydrogels
2.3. Crowding Effect
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Quartz Crystal Microbalance with Dissipation (QCM-D)
4.3. Preparation of XG/CNC Mixtures and the Investigation of Their Gelling Properties by the Inverted Test Tube Method
4.4. Enzymatic Degalactosylation of Xyloglucan
4.5. Rheology
4.6. Osmotic Dehydration
4.7. Molding Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Moreau, C.; Villares, A.; Capron, I.; Cathala, B. Tuning Supramolecular Interactions of Cellulose Nanocrystals to Design Innovative Functional Materials. Ind. Crop. Prod. 2016, 93, 96–107. [Google Scholar] [CrossRef]
- Cerclier, C.V.; Guyomard-Lack, A.; Cousin, F.; Jean, B.; Bonnin, E.; Cathala, B.; Moreau, C. Xyloglucan-Cellulose Nano-crystal Multilayered Films: Effect of Film Architecture on Enzymatic Hydrolysis. Biomacromolecules 2013, 14, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Quemener, B.; Bonnin, E.; Alvarado, C.; Bouchet, B.; Villares, A.; Moreau, C.; Cathala, B. Exploring Architec-ture of Xyloglucan Cellulose Nanocrystal Complexes through Enzyme Susceptibility at Different Adsorption Regimes. Biomacromolecules 2015, 16, 589–596. [Google Scholar] [CrossRef]
- Leray, N.; Talantikite, M.; Villares, A.; Cathala, B. Xyloglucan-Cellulose Nanocrystal-Chitosan Double Network Hy-drogels for Soft Actuators. Carbohydr. Polym. 2022, 293, 119753. [Google Scholar] [CrossRef]
- Villares, A.; Moreau, C.; Dammak, A.; Capron, I.; Cathala, B. Kinetic Aspects of the Adsorption of Xyloglucan onto Cellulose Nanocrystals. Soft Matter 2015, 11, 6472–6481. [Google Scholar] [CrossRef]
- Hayashi, K.; Maclachlan, G. Pea Xyloglucan and Cellulose. Plant Physiol. 1984, 75, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. A Revised Architecture of Primary Cell Walls Based on Biomechanical Changes Induced by Substrate-Specific Endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Mimura, M.; Kajiwara, K. Diversity and Versatility of Plant Seed Xyloglucan. Trends Glycosci. Glycotechnol. 2002, 14, 355–376. [Google Scholar] [CrossRef][Green Version]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O. Langer. Self-Assembled Hydrogels Utilizing Pol-ymer-Nanoparticle Interactions. Nat. Commun. 2015, 6, 9. [Google Scholar] [CrossRef]
- Hu, Z.; Cranston, E.D.; Ng, R.; Pelton, R. Tuning Cellulose Nanocrystal Gelation with Polysaccharides and Surfactants. Langmuir 2014, 30, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Morozova, S.M.; Gevorkian, A.; Kumacheva, E. Design, Characterization and Applications of Nanocol-loidal Hydrogels. Chem. Soc. Rev. 2023, 52, 5317–5339. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, K.; Ren, Q.; Chen, G.; Wei, P.; Zhu, M. Nanoparticle–Polymer Synergies in Nano-composite Hydrogels: From Design to Application. Macromol. Rapid Commun. 2018, 39, 1800337. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle Solutions as Adhesives for Gels and Biological Tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Spalla, O. Nanoparticle Interactions with Polymers and Polyelectrolytes. Curr. Opin. Colloid Interface Sci. 2002, 7, 179–185. [Google Scholar] [CrossRef]
- Spalla, O.; Cabane, B. Growth of Colloidal Aggregates through Polymer Bridging. Colloid Polym. Sci. 1993, 271, 357–371. [Google Scholar] [CrossRef]
- Spalla, O.; Nabavi, M.; Minter, J.; Cabane, B. Osmotic Compression of Mixtures of Polymers and Particles. Colloid Polym. Sci. 1996, 274, 555–567. [Google Scholar] [CrossRef]
- Haraguchi, K. Nanocomposite Hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Hayashi, K.; Mardsen, M.P.F.; Delmar, D.P. Pea Xyloglucan and Cellulose: Xyloglucan-Cellulose Interactions in Vitro and in Vivo. Plant Physiol. 1987, 83, 384–389. [Google Scholar] [CrossRef]
- Hayashi, T.; Kaida, R. Functions of Xyloglucan in Plant Cells. Mol. Plant 2011, 4, 17–24. [Google Scholar] [CrossRef]
- Benselfelt, T.; Cranston, E.D.; Ondaral, S.; Johansson, E.; Brumer, H.; Rutland, M.W.; Wågberg, L. Adsorption of Xyloglucan onto Cellulose Surfaces of Different Morphologies: An Entropy-Driven Process. Biomacromolecules 2016, 17, 2801–2811. [Google Scholar] [CrossRef]
- Kishani, S.; Vilaplana, F.; Ruda, M.; Hansson, P.; Wågberg, L. The Influence of Solubility on the Ad-sorption of Different Xyloglucan Fractions at Cellulose Water Interfaces. Biomacromolecules 2020, 21, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung Von Schwingquarzen Zur Wagung Dunner Schichten Und Zur Mikrowagung. Z. Fur Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous Frequency and Dissipation Factor Qcm Measurements of Biomolecular Adsorption and Cell Adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Parikka, K.; Tenkanen, M. Oxidation of Methyl Alpha-D-Galactopyranoside by Galactose Oxidase: Products Formed and Optimization of Reaction Conditions for Production of Aldehyde. Carbohydr. Res. 2009, 344, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.; Parikka, K.; Sontag-Strohm, T.; Österberg, M.; Tenkanen, M.; Mikkonen, K.S. Strength-ening Effect of Nanofibrillated Cellulose Is Dependent on Enzymatically Oxidized Polysaccharide Gel Matrices. Eur. Polym. J. 2015, 71, 171–184. [Google Scholar] [CrossRef]
- Parikka, K.; Leppänen, A.-S.; Pitkänen, L.; Reunanen, M.; Willför, S.; Tenkanen, M. Oxidation of Polysaccharides by Galactose Oxidase. J. Agric. Food Chem. 2009, 58, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Yamatoya, K.; Nishinari, K. Tailoring of Xyloglucan Properties Using an Enzyme. Food Hydrocoll. 1998, 12, 25–28. [Google Scholar] [CrossRef]
- Yamanaka, S.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K.; Shirakawa, M.; Yamatoya, K. Gelation of Enzymatically Degraded Xyloglucan Extracted from Tamarind Seed. Sen’i Gakkaishi 1999, 55, 528–532. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Narita, T.; Ducouret, G.; Merten, O.-W. Study on the Sol-Gel Transition of Xyloglucan Hydrogels. Carbohydr. Polym. 2010, 80, 555–562. [Google Scholar] [CrossRef]
- Desbrières, J.; Hirrien, M.; Rinaudo, M. A Calorimetric Study of Methylcellulose Gelation. Carbohydr. Polym. 1998, 37, 145–152. [Google Scholar] [CrossRef]
- Heymann, E. Studies on Sol-Gel Transformations. I. The Inverse Sol-Gel Transformation of Methylcellulose in Water. Trans. Faraday Soc. 1935, 31, 846–864. [Google Scholar] [CrossRef]
- Hourdet, D.; Gadgil, J.; Podhajecka, K.; Badiger, M.V.; Brulet, A.; Wadgaonkar, P.P. Thermoreversible Behavior of Asso-ciating Polymer Solutions: Thermothinning Versus Thermothickening. Macromolecules 2005, 38, 8512–8521. [Google Scholar] [CrossRef]
- de Freitas, R.A.; Spier, V.C.; Sierakowski, M.R.; Nicolai, T.; Benyahia, L.; Chassenieux, C. Transient and Qua-si-Permanent Networks in Xyloglucan Solutions. Carbohydr. Polym. 2015, 129, 216–223. [Google Scholar] [CrossRef]
- Talantikite, M.; Stimpson, T.C.; Gourlay, A.; Le-Gall, S.; Moreau, C.; Cranston, E.D.; Moran-Mirabal, J.M.; Cathala, B. Bi-oinspired Thermoresponsive Xyloglucan-Cellulose Nanocrystal Hydrogels. Biomacromolecules 2021, 22, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Bonnet-Gonnet, C.; Belloni, L.; Cabane, B. Osmotic Pressure of Latex Dispersions. Langmuir 1994, 10, 4012–4021. [Google Scholar] [CrossRef]
- Cohen, J.A.; Podgornik, R.; Hansen, P.L.; Parsegian, V.A. A Phenomenological One-Parameter Equation of State for Osmotic Pressures of PEG and Other Neutral Flexible Polymers in Good Solvents. J. Phys. Chem. B 2009, 113, 3709–3714. [Google Scholar] [CrossRef]
- Bouchoux, A.; Cayemitte, P.-E.; Jardin, J.; Gésan-Guiziou, G.; Cabane, B. Casein Mi-celle Dispersions under Osmotic Stress. Biophys. J. 2009, 96, 693–706. [Google Scholar] [CrossRef]
- Antoine, C.A.; Cassan, D.; Carvajal-Millan, E.; Bouchoux, A.; Micard, V. Making Dense Covalent Arabinoxylan Gels with High Swelling Properties: A Strategy Based on Water Extraction through Osmotic Com-pression. ACS Appl. Polym. Mater. 2021, 3, 6176–6185. [Google Scholar] [CrossRef]
- Guccini, V.; Phiri, J.; Trifol, J.; Rissanen, V.; Mousavi, S.M.; Vapaavuori, J.; Tammelin, T.; Maloney, T.; Kontturi, E. Tuning the Porosity, Water Interaction, and Redispersion of Nanocellulose Hydrogels by Osmotic Dehydration. ACS Appl. Polym. Mater. 2021, 4, 24–28. [Google Scholar] [CrossRef]
- Guccini, V.; Yu, S.; Agthe, M.; Gordeyeva, K.; Trushkina, Y.; Fall, A.; Schütz, C.; Salazar-Alvarez, G. Inducing Nematic Ordering of Cellulose Nanofibers Using Osmotic Dehydration. Nanoscale 2018, 10, 23157–23163. [Google Scholar] [CrossRef] [PubMed]
- Colby, R.H. Structure and Linear Viscoelasticity of Flexible Polymer Solutions: Comparison of Polyelectrolyte and Neu-tral Polymer Solutions. Rheol. Acta 2010, 49, 425–442. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Nonlinear Viscoelastic Characterization of Charged Cellulose Nanocrystal Network Structure In the Presence of Salt in Aqueous Me-dia. Cellulose 2020, 27, 5729–5743. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Huang, J.; Wu, D.; Lv, Q. Rheological Properties of Nanocrystalline Cellulose Suspensions. Carbohydr. Polym. 2017, 157, 303–310. [Google Scholar] [CrossRef]
- Mandin, S.; Moreau, S.; Talantikite, M.; Novalès, B.; Maigret, J.-E.; Cathala, B.; Moreau, C. Cellulose Nanofibrils/Xyloglucan Bio-Based Aerogels with Shape Recovery. Gels 2021, 7, 5. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, G.; Moreau, C.; Villares, A.; Chassenieux, C.; Cathala, B. Xyloglucan–Cellulose Nanocrystals Mixtures: A Case Study of Nanocolloidal Hydrogels and Levers for Tuning Functional Properties. Gels 2024, 10, 334. https://doi.org/10.3390/gels10050334
Rangel G, Moreau C, Villares A, Chassenieux C, Cathala B. Xyloglucan–Cellulose Nanocrystals Mixtures: A Case Study of Nanocolloidal Hydrogels and Levers for Tuning Functional Properties. Gels. 2024; 10(5):334. https://doi.org/10.3390/gels10050334
Chicago/Turabian StyleRangel, Géraldine, Céline Moreau, Ana Villares, Christophe Chassenieux, and Bernard Cathala. 2024. "Xyloglucan–Cellulose Nanocrystals Mixtures: A Case Study of Nanocolloidal Hydrogels and Levers for Tuning Functional Properties" Gels 10, no. 5: 334. https://doi.org/10.3390/gels10050334
APA StyleRangel, G., Moreau, C., Villares, A., Chassenieux, C., & Cathala, B. (2024). Xyloglucan–Cellulose Nanocrystals Mixtures: A Case Study of Nanocolloidal Hydrogels and Levers for Tuning Functional Properties. Gels, 10(5), 334. https://doi.org/10.3390/gels10050334






