The MnO2/GelMA Composite Hydrogels Improve the ROS Microenvironment of Annulus Fibrosus Cells by Promoting the Antioxidant and Autophagy through the SIRT1/NRF2 Pathway
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of MnO2 Nanoparticles and Composite Hydrogels
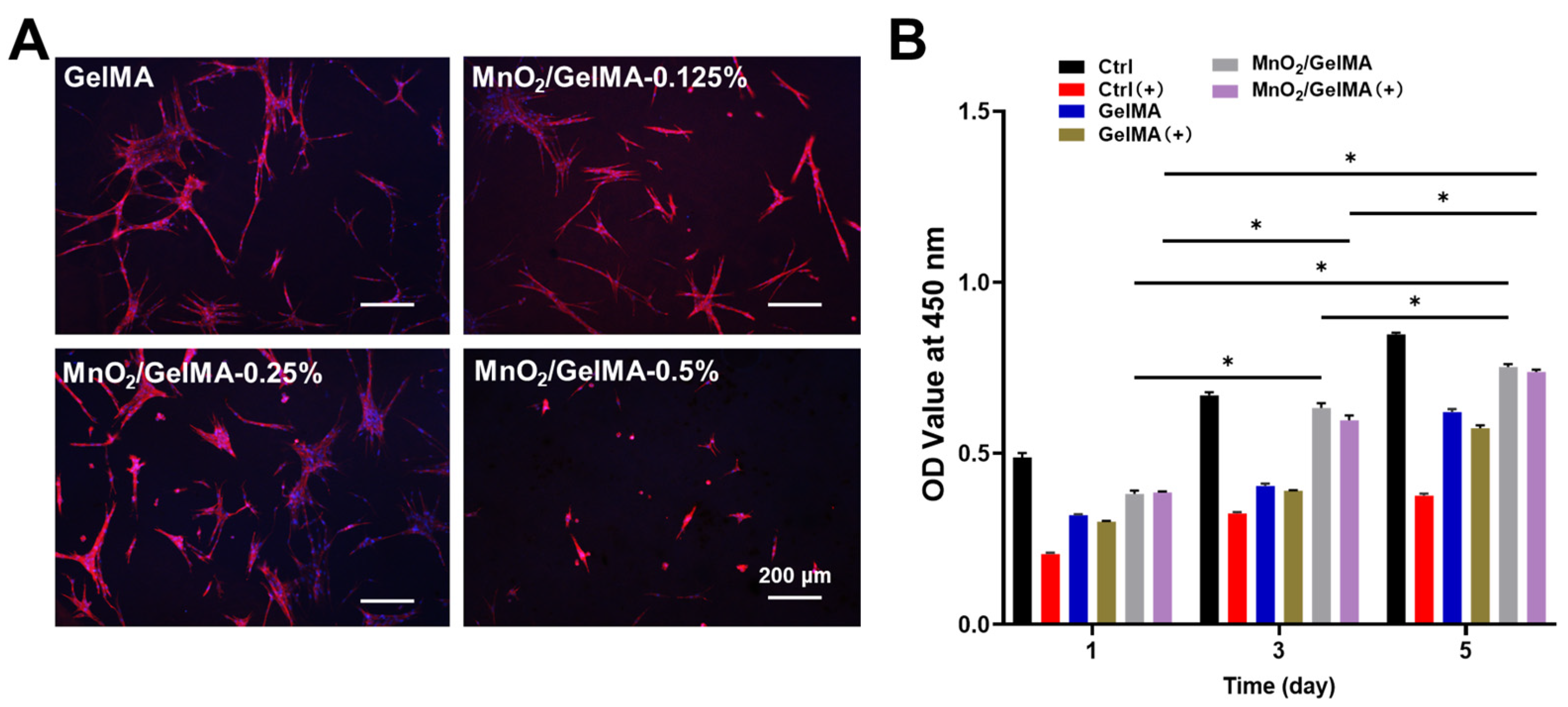
2.2. Biocompatibility of MnO2/GelMA Composite Hydrogels
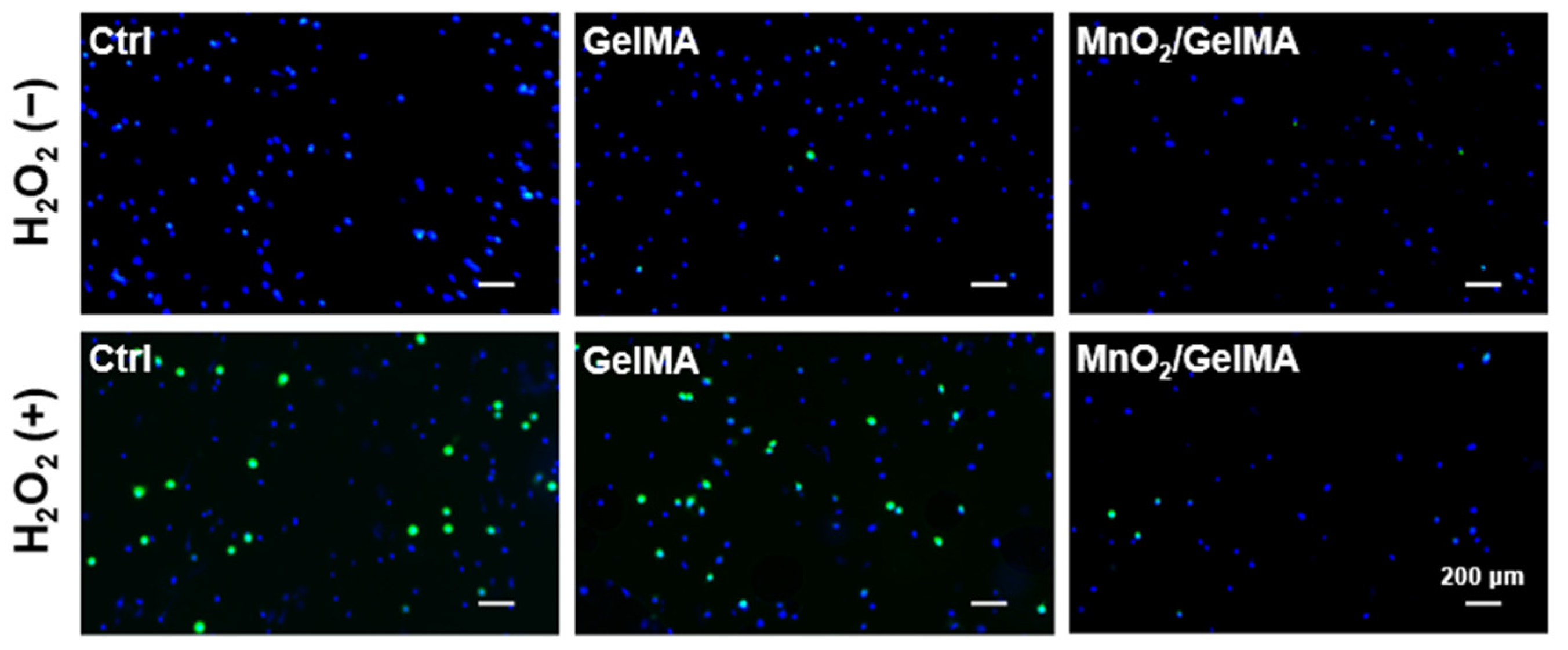
2.3. Scavenging Effect of Composite Hydrogels on Intracellular ROS
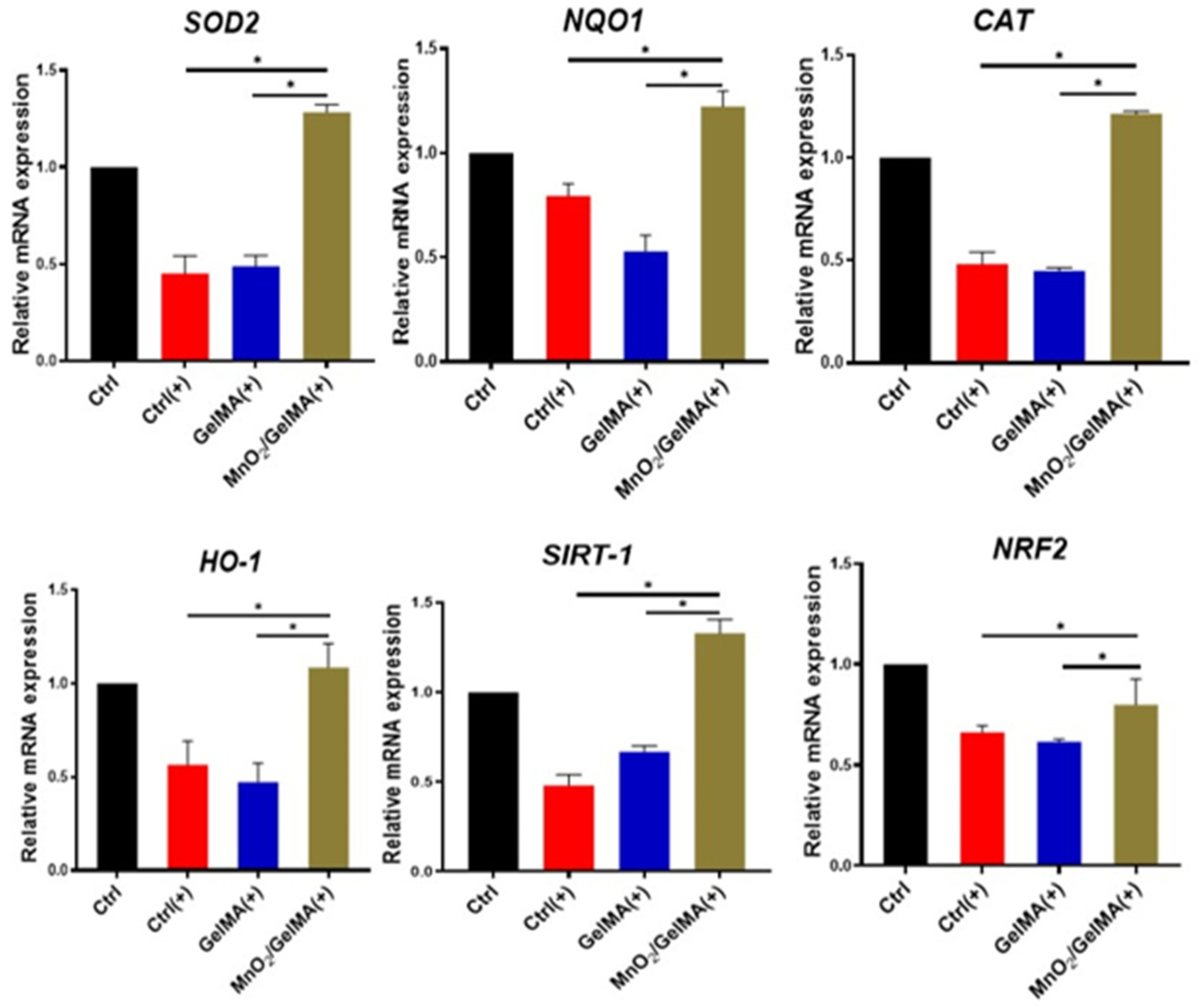
2.4. Antioxidant Effects of MnO2/GelMA Composite Hydrogels on AFCs
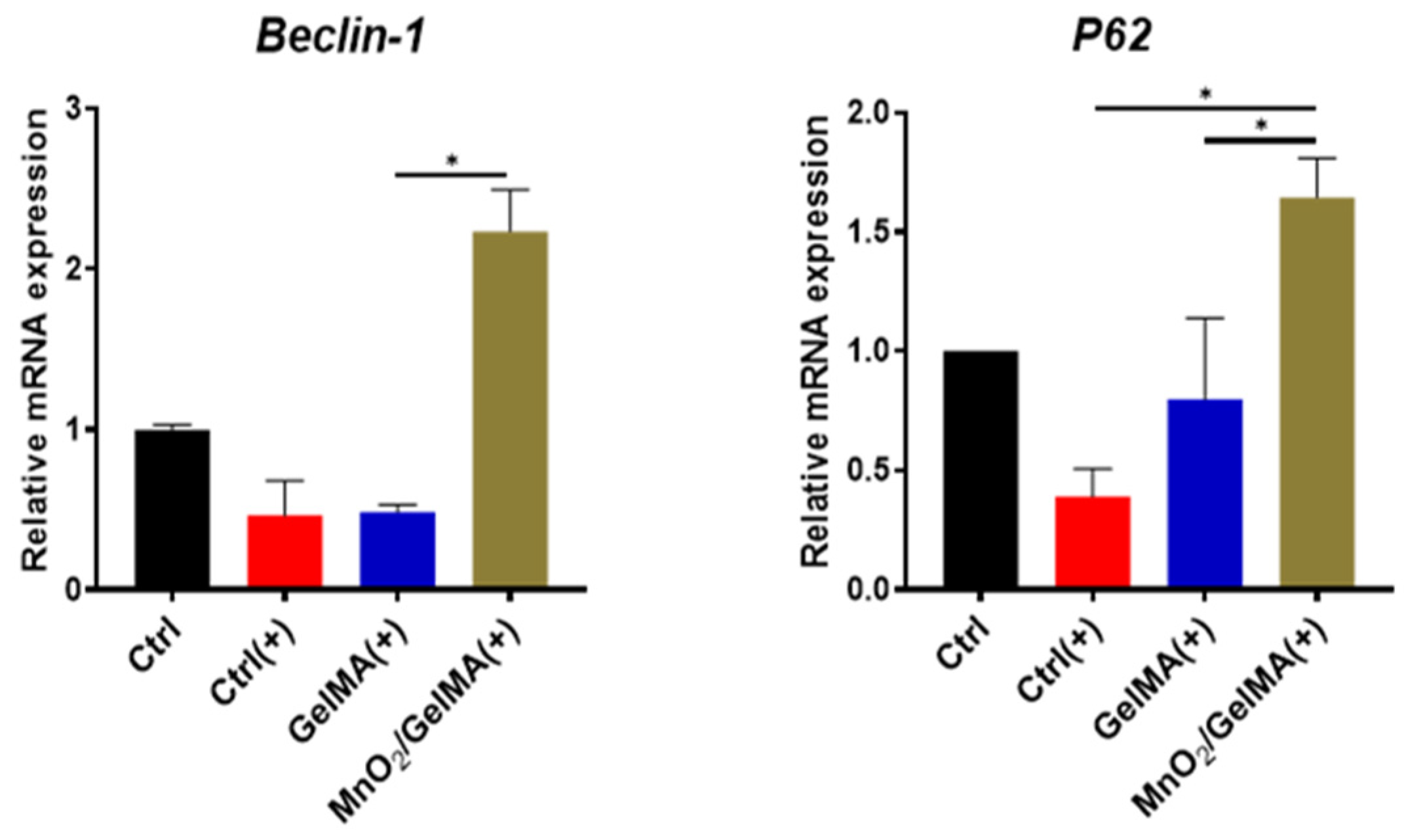
2.5. Effects of Composite Hydrogels in Promoting Cellular Autophagy
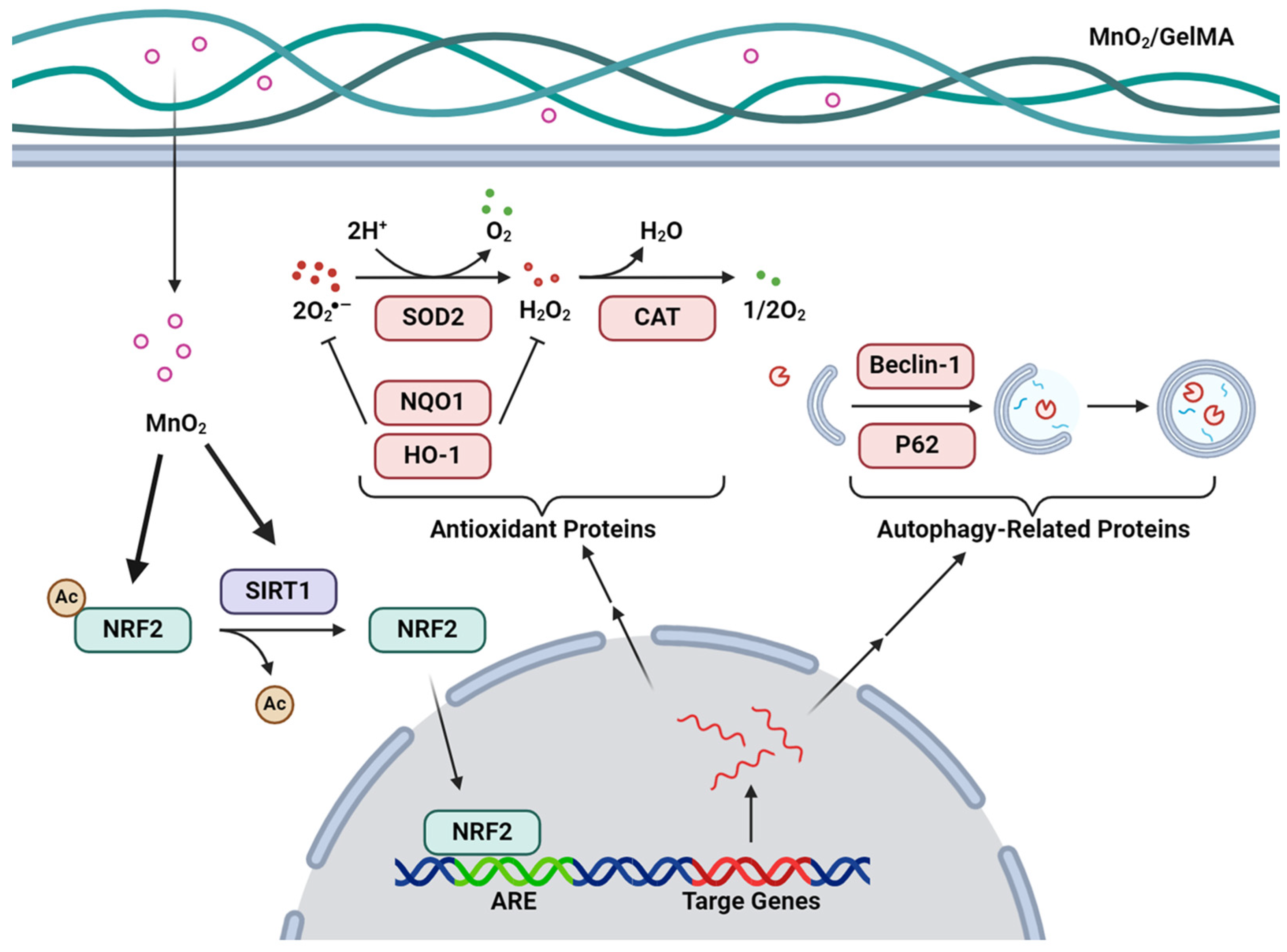
2.6. Discussion of Findings
3. Conclusions
4. Materials and Methods
4.1. Preparation of MnO2 Nanoparticles
4.2. Preparation of MnO2/GelMA Composite Hydrogels
4.3. Morphology of MnO2 Nanoparticles and Composite Hydrogels
4.4. Cell Viability and Proliferation Measurements
4.5. Cytoskeleton Staining
4.6. Mechanical Properties of Composite Hydrogels
4.7. Detection of Intracellular ROS
4.8. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.9. Western Blotting Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Jin, Z.; Wang, D.; Zhang, H.; Liang, J.; Feng, X.; Zhao, J.; Sun, L. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: Results from the global burden of disease study 2017. Ann. Rheum. Dis. 2020, 79, 1014–1022. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Wang, Y.; Zheng, Z.; Wang, S.; Na, S.; Zhang, S. Treatment of Intervertebral Disc Degeneration. Orthop. Surg. 2022, 14, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Cai, K.; Cai, X.; Zhang, K.; Qiu, R.; Jiang, G.; Luo, K. Recent advances in the repair of degenerative intervertebral disc for preclinical applications. Front. Bioeng. Biotechnol. 2023, 11, 1259731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, H.; Zheng, Z.; Wu, D. Design principles in mechanically adaptable biomaterials for repairing annulus fibrosus rupture: A review. Bioact. Mater. 2024, 31, 422–439. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Guo, C.; Huang, Z.; Zhang, W.; Ma, F.; Wang, Z.; Kong, Q.; Wang, Y. Application and development of hydrogel biomaterials for the treatment of intervertebral disc degeneration: A literature review. Front. Cell Dev. Biol. 2023, 11, 1286223. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Bilgen, B.; Hasirci, N.; Hasirci, V. Anatomical meniscus construct with zone specific biochemical composition and structural organization. Biomaterials 2019, 218, 119361. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Deng, R.; Yan, H.; Liu, X.; Kang, R. Intervertebral disc degeneration and inflammatory microenvironment: Expression, pathology, and therapeutic strategies. Inflamm. Res. 2023, 72, 1811–1828. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, H.; Yang, R.; Hou, Y.; Li, Y.; Zhu, J.; Fu, C. Biomaterials delivery strategies to repair degenerated intervertebral discs by regulating the inflammatory microenvironment. Front. Immunol. 2023, 14, 1051606. [Google Scholar] [CrossRef]
- Zhou, H.; He, J.; Liu, R.; Cheng, J.; Yuan, Y.; Mao, W.; Zhou, J.; He, H.; Liu, Q.; Tan, W.; et al. Microenvironment-responsive metal-phenolic network release platform with ROS scavenging, anti-pyroptosis, and ECM regeneration for intervertebral disc degeneration. Bioact. Mater. 2024, 37, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhu, D.; Wang, Y.; Miao, Z.; Chen, Z.; Lin, Z.; Lin, J.; Huang, C.; Pan, L.; Wang, L.; et al. Targeting STING attenuates ROS induced intervertebral disc degeneration. Osteoarthr. Cartil. 2021, 29, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.G.; Boos, N.; Wiest, I.; Aebi, M. Immunolocalization of major interstitial collagen types in human lumbar intervertebral discs of various ages. Virchows Arch. 1998, 432, 67–76. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.; Wang, X.; Li, Z.; Zhang, J.; Pei, Y.; Zheng, Z.; Wang, J. Melatonin activates autophagy via the NF-kappaB signaling pathway to prevent extracellular matrix degeneration in intervertebral disc. Osteoarthr. Cartil. 2020, 28, 1121–1132. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Wang, D.; Peng, P.; Xu, X.; Gao, B.; Zheng, C.; Wang, H.; Jia, H.; Shang, Q.; et al. Quercetin Suppresses Apoptosis and Attenuates Intervertebral Disc Degeneration via the SIRT1-Autophagy Pathway. Front. Cell Dev. Biol. 2020, 8, 613006. [Google Scholar] [CrossRef]
- Wang, D.K.; Zheng, H.L.; Zhou, W.S.; Duan, Z.W.; Jiang, S.D.; Li, B.; Zheng, X.F.; Jiang, L.S. Mitochondrial Dysfunction in Oxidative Stress-Mediated Intervertebral Disc Degeneration. Orthop. Surg. 2022, 14, 1569–1582. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, e2101526. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Liu, L.; Wang, M.; Li, G.; Li, H.; Li, B.; Yu, S.; Ma, D.; Xue, W. Biodegradable Hollow Polydopamine@manganese Dioxide as an Oxygen Self-Supplied Nanoplatform for Boosting Chemo-photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 57009–57022. [Google Scholar] [CrossRef]
- Chang, C.C.; Dinh, T.K.; Lee, Y.A.; Wang, F.N.; Sung, Y.C.; Yu, P.L.; Chiu, S.C.; Shih, Y.C.; Wu, C.Y.; Huang, Y.D.; et al. Nanoparticle Delivery of MnO2 and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 44407–44419. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhao, W.; Jiang, K.; He, J.; Chen, L.; Cui, W.; Li, Y. Progress in regulating inflammatory biomaterials for intervertebral disc regeneration. Bioact. Mater. 2024, 33, 506–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Hu, Z.L.; Qi, Y.H.; Li, H.Y.; Chang, X.; Gao, X.X.; Liu, C.H.; Li, Y.Y.; Lou, J.H.; Zhai, Y.; et al. Pretreatment of nucleus pulposus mesenchymal stem cells with appropriate concentration of H2O2 enhances their ability to treat intervertebral disc degeneration. Stem Cell Res. Ther. 2022, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, G.; Wang, Y.; Lin, X.; Zhou, J.; Wang, Z.; Suo, N. Dimethyl fumarate protects nucleus pulposus cells from inflammation and oxidative stress and delays the intervertebral disc degeneration. Exp. Ther. Med. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shi, Y.; Jiang, L.; Bu, W.; Zhang, K.; Lin, W.; Pan, C.; Xu, Z.; Du, J.; Chen, H.; et al. N-Acetylcysteine-Derived Carbon Dots for Free Radical Scavenging in Intervertebral Disc Degeneration. Adv. Healthc. Mater. 2023, 12, e2300533. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ouyang, Y.; Zhao, T.; Wang, Z.; Yan, Q.; Qian, Q.; Wang, W.; Wang, S. Antioxidant Hydrogels: Antioxidant Mechanisms, Design Strategies, and Applications in the Treatment of Oxidative Stress-Related Diseases. Adv. Healthc. Mater. 2024, 13, e2303817. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Bahrampour, S.; Mozafari, M.; Gasik, M. Surface functionalization of anodized tantalum with Mn3O4 nanoparticles for effective corrosion protection in simulated inflammatory condition. Ceram. Int. 2022, 48, 3148–3156. [Google Scholar] [CrossRef]
- Kawai, Y.; Garduno, L.; Theodore, M.; Yang, J.; Arinze, I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011, 286, 7629–7640. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, C.; Jiang, H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021, 65, 101207. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Li, N.; Zhang, F.; Yu, P.; Meng, Q. Resveratrol Suppresses Annulus Fibrosus Cell Apoptosis through Regulating Oxidative Stress Reaction in an Inflammatory Environment. Biomed. Res. Int. 2021, 2021, 9100444. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Han, F.; Yuan, Z.; Zhu, Z.; Liu, C.; Tu, Z.; Guo, Q.; Zhao, R.; Zhang, W.; Wang, H.; et al. Fucoidan-loaded nanofibrous scaffolds promote annulus fibrosus repair by ameliorating the inflammatory and oxidative microenvironments in degenerative intervertebral discs. Acta Biomater. 2022, 148, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, Y.; Yurube, T.; Kakutani, K.; Takada, T.; Ito, M.; Takeoka, Y.; Kanda, Y.; Miyazaki, S.; Kuroda, R.; Nishida, K. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthr. Cartil. 2019, 27, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, L.; Al Mamun, A.; Ni, L.; Gao, W.; Lin, Y.; Jin, H.; Zhang, X.; Wang, X. The therapeutic effect of TBK1 in intervertebral disc degeneration via coordinating selective autophagy and autophagic functions. J. Adv. Res. 2021, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lamark, T.; Sjottem, E.; Larsen, K.B.; Awuh, J.A.; Overvatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011, 7, 279–296. [Google Scholar] [CrossRef]
- Yurube, T.; Takeoka, Y.; Kanda, Y.; Kuroda, R.; Kakutani, K. Intervertebral disc cell fate during aging and degeneration: Apoptosis, senescence, and autophagy. N. Am. Spine Soc. J. 2023, 14, 100210. [Google Scholar] [CrossRef]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar]
- Khaleque, M.A.; Kim, J.H.; Lee, H.H.; Kim, G.H.; You, W.Y.; Lee, W.J.; Kim, Y.Y. Comparative Analysis of Autophagy and Apoptosis in Disc Degeneration: Understanding the Dynamics of Temporary-Compression-Induced Early Autophagy and Sustained-Compression-Triggered Apoptosis. Int. J. Mol. Sci. 2024, 25, 2352. [Google Scholar] [CrossRef]
- Shen, C.; Yan, J.; Jiang, L.S.; Dai, L.Y. Autophagy in rat annulus fibrosus cells: Evidence and possible implications. Arthritis Res. Ther. 2011, 13, R132. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Kong, L.; Liu, C.; Xu, H.G. Human Bone Marrow Mesenchymal Stem Cell-derived Exosomes Attenuate IL-1beta-induced Annulus Fibrosus Cell Damage. Am. J. Med. Sci. 2020, 360, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Shen, H.; Wang, W.; Lu, H.; Jiang, L. TGF-beta1 reduces the oxidative stress-induced autophagy and apoptosis in rat annulus fibrosus cells through the ERK signaling pathway. J. Orthop. Surg. Res. 2019, 14, 241. [Google Scholar] [CrossRef] [PubMed]



| Reagent | Volume |
|---|---|
| Oligo (dT)18 Primer | 1 μL |
| RNA | 1/X * |
| 5X All-in-one RT MasterMix | 4 μL |
| Nuclease-free H2O | 15-1/X |
| Target Genes * | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| CAT | GGCCTGACTGACGCGATTGC | CTGCTCCTTCCACTGCTTCATCTG |
| SOD2 | GCTGGAGGCTATCAAGCGTGAC | TTAGAGCAGGCGGCAATCTGTAAG |
| SIRT1 | GCTCGCCTTGCTGTGGACTTC | GTGACACAGAGATGGCTGGAACTG |
| HO-1 | ACAGACAGAGTTTCTTCGCC | ATAAATTCCCACTGCCACGG |
| NRF2 | GCAGGCTGAGACTACCACTG | CTGGCATCATCCGTGGAGAG |
| NQO1 | GAAGAGCACTGATCGTACTGGC | GGATACTGAAAGTTCGCAGGG |
| Beclin-1 | GGGTCTAAGGCGTCCAGCAG | GACACCATCCTGGCGAGTTT |
| P62 | TATTACAGCCAGAGTCAAGG | CTACATACAGAAGCCAGAATG |
| GAPDH | GACCTGACCTGCCGTCTA | AGGAGTGGGTGTCGCTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Huang, M.; Li, J.; Meng, Q.; Hu, J.; Chen, Q.; He, H.; Jiang, H.; Han, F.; Meng, B.; et al. The MnO2/GelMA Composite Hydrogels Improve the ROS Microenvironment of Annulus Fibrosus Cells by Promoting the Antioxidant and Autophagy through the SIRT1/NRF2 Pathway. Gels 2024, 10, 333. https://doi.org/10.3390/gels10050333
Xu B, Huang M, Li J, Meng Q, Hu J, Chen Q, He H, Jiang H, Han F, Meng B, et al. The MnO2/GelMA Composite Hydrogels Improve the ROS Microenvironment of Annulus Fibrosus Cells by Promoting the Antioxidant and Autophagy through the SIRT1/NRF2 Pathway. Gels. 2024; 10(5):333. https://doi.org/10.3390/gels10050333
Chicago/Turabian StyleXu, Bohan, Mingxuan Huang, Jiaying Li, Qingchen Meng, Jie Hu, Qianglong Chen, Hui He, Hao Jiang, Fengxuan Han, Bin Meng, and et al. 2024. "The MnO2/GelMA Composite Hydrogels Improve the ROS Microenvironment of Annulus Fibrosus Cells by Promoting the Antioxidant and Autophagy through the SIRT1/NRF2 Pathway" Gels 10, no. 5: 333. https://doi.org/10.3390/gels10050333
APA StyleXu, B., Huang, M., Li, J., Meng, Q., Hu, J., Chen, Q., He, H., Jiang, H., Han, F., Meng, B., & Liang, T. (2024). The MnO2/GelMA Composite Hydrogels Improve the ROS Microenvironment of Annulus Fibrosus Cells by Promoting the Antioxidant and Autophagy through the SIRT1/NRF2 Pathway. Gels, 10(5), 333. https://doi.org/10.3390/gels10050333







