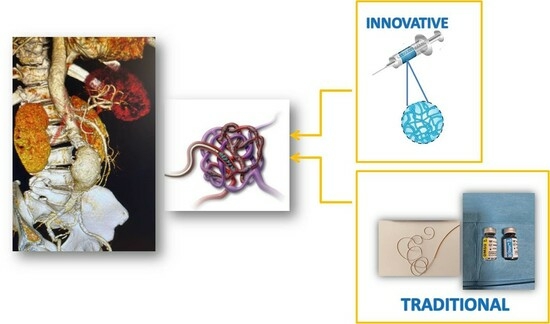
OnyxTMGel or Coil versus Hydrogel as Embolic Agents in Endovascular Applications: Review of the Literature and Case Series
Abstract
:1. Introduction
2. Liquid Embolic Agent
OnyxTM Gel
3. Coil Embolic Agent
4. Hydrogel Embolic Agents
4.1. Chitosan Hydrogel
4.2. Alginate Hydrogel
4.3. Silk-Elastin-like Protein Hydrogel
4.4. Polaxamer 407-Based Hydrogel
5. Endovascular Application of Embolic Agents
5.1. Aneurysm
5.2. Arteriovenous Malformation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Clinical Case 1: Right Renal Visceral Arteriovenous Malformation




Appendix A.2. Clinical Case 2: Treatment of Endoleak Type II Inferior Mesenteric Artery



Appendix A.3. Clinical Case 3: Splenic Visceral Aneurysm




Appendix A.4. Clinical Case 4: Onyx Use in Endoleak Type 1 Excision Post EVAR ABI

References
- Li, X.; Ullah, M.W.; Li, B.; Chen, H. Recent Progress in Advanced Hydrogel-Based Embolic Agents: From Rational Design Strategies to Improved Endovascular Embolization. Adv. Healthc. Mater. 2023, 12, 2202787. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.B.R.; Rebouças, J.C.; Nunes, S.L.O.; de Luccia, N.; Pereira, R.M.R. Successful endovascular correction of common carotid pseudoaneurysm secondary to Behçet’s disease: Case report and review of the literature. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S127), S94–S97. [Google Scholar]
- Rodriguez-Calienes, G.; Saal-Zapata, N.F.; Borjas-Calderón, L.E.; Alvarez-Trujillo, R. Rodríguez-Varela. Curative embolization for pediatric Spetzler-Martin grade iii cerebral arteriovenous malformations. World Neurosurg. 2022, 160, e494. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Abo, D.; Kinota, N.; Soyama, T.; Takahashi, B.; Yoshino, Y.; Tsuneta, S.; Kudo, K. Successful transvenous embolization for type II uterine arteriovenous malformation: A case report. Radiol. Case Rep. 2021, 16, 2007. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hou, P. Sac Embolization and Side Branch Embolization for Preventing Type II Endoleaks After Endovascular Aneurysm Repair: A Meta-analysis MD. J. Endovasc. Ther. 2020, 27, 109–116. [Google Scholar] [CrossRef]
- Goertz, L.; Dorn, F.; Kraus, B.; Borggrefe, J.; Schlamann, M.; Forbrig, R.; Kabbasch, C. Safety and efficacy of the Derivo Embolization Device for the treatment of ruptured intracranial aneurysms. J. Neurointerv. Surg. 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, S.; Yunaiyama, D.; Saguchi, T.; Otaka, J.; Yamada, T.; Ito, H.; Sugimoto, K.; Itoi, T.; Saito, K. A case of a patient who underwent transcatheter arterial embolization for unruptured splenic aneurysm during pregnancy. Radiol. Case Rep. 2021, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M.; Lim, B.B.; Kim, Y.R.; Jeon, G.S.; Jung, S.H. Transcatheter arterial embolization in the management of postpartum hemorrhage due to genital tract injury after vaginal delivery. J. Vasc. Interv. Radiol. 2021, 32, 99. [Google Scholar] [CrossRef]
- Wang, C.; Hu, J.; Sheth, R.; Oklu, R. Emerging embolic agents in endovascular embolization: An overview. Prog. Biomed. Eng. 2020, 2, 012003. [Google Scholar] [CrossRef]
- Tiralongo, F.; Distefano, G.; Palermo, M.; Granata, A.; Giurazza, F.; Vacirca, F.; Palmucci, S.; Venturini, M.; Basile, A. Liquid and Solid Embolic Agents in Gonadal Veins. J. Clin. Med. 2021, 10, 1596. [Google Scholar] [CrossRef]
- Cassano, R.; Perri, P.; Esposito, A.; Intrieri, F.; Sole, R.; Curcio, F.; Trombino, S. Expanded Polytetrafluoroethylene Membranes for Vascular Stent Coating: Manufacturing, Biomedical and Surgical Applications, Innovations and Case Reports. Membranes 2023, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Perri, P.; Piro, P.; Galassi, S.; Sole, R.; Trombino, S.; Cassano, R. Synthetic Haemostatic Sealants: Effectiveness, Safety, and In Vivo Applications. Pharmaceuticals 2024, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bhattacharya, P.; Neogi, S. Bioadhesives in Biomedical Applications: A Critical Review. In Progress in Adhesion and Adhesives; Mittal, K.L., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; Volume 6, pp. 131–154. [Google Scholar]
- Lord, J.; Britton, H.; Spain, S.G.; Lewis, A.L. Advancements in the Development on New Liquid Embolic Agents for Use in Therapeutic Embolisation. J. Mater. Chem. B 2020, 8, 8207–8218. [Google Scholar] [CrossRef] [PubMed]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, L.; An, Q.; Chen, L.; Wen, Y.; Fang, F.; Zhu, W.; Yi, T. Novel Hydrogel Material as a Potential Embolic Agent in Embolization Treatments. Sci. Rep. 2016, 6, 32145. [Google Scholar] [CrossRef]
- Trombino, S.; Sole, R.; Curcio, F.; Cassano, R. Polymeric Based Hydrogel Membranes for Biomedical Applications. Membranes 2023, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Curcio, F.; Cassano, R.; Curcio, M.; Cirillo, G.; Iemma, F. Polymeric Biomaterials for the Treatment of Cardiac Post-Infarction Injuries. Pharmaceutics 2021, 13, 1038. [Google Scholar] [CrossRef]
- Cassano, R.; Curcio, F.; Sole, R.; Trombino, S. Chapter 3: Hydrogel based on hyaluronic acid. In Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 35–46. [Google Scholar]
- Li, M.; Jin, M.; Yang, H. Remodelers of the vascular microenvironment: The effect of biopolymeric hydrogels on vascular diseases. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130764. [Google Scholar] [CrossRef]
- Pal, A.; Blanzy, J.; Gómez, K.J.R.; Preul, M.C.; Vernon, B.L. Liquid Embolic Agents for Endovascular Embolization: A Review. Gels 2023, 9, 378. [Google Scholar] [CrossRef]
- Piacentino, F.; Fontana, F.; Curti, M.; Macchi, E.; Coppola, A.; Ossola, C.; Giorgianni, A.; Marra, P.; Mosconi, C.; Ierardi, A.M.; et al. Non-Adhesive Liquid Embolic Agents in Extra-Cranial District: State of the Art and Review of the Literature. J. Clin. Med. 2021, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Albadawi, H.; Akbari, M.; Zhang, Y.S.; Duggan, M.J.; Sahani, D.V.; Olsen, B.D.; Khademhosseini, A.; Oklu, R. An injectable shear-thinning biomaterial for endovascular embolization. Sci. Transl. Med. 2016, 8, 365ra156. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.K.; Vora, L.K.; Umeyor, C.; Surve, D.; Patel, A.; Biswas, S.; Patel, K.; Patravale, V.B. Polymeric in situ forming depots for long-acting drug delivery systems. Adv. Drug Deliv. Rev. 2023, 200, 115003. [Google Scholar] [CrossRef]
- Hobohm, L.; Keller, K.; Münzel, T.; Gori, T.; Konstantinides, S.V. EkoSonic® endovascular system and other catheter-directed treatment reperfusion strategies for acute pulmonary embolism: Overview of efficacy and safety outcomes. Expert Rev. Med. Devices 2020, 17, 739–749. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2022, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, G.; Lu, J.; Miao, G.; Liang, D.; Wang, J.; Wei, L.; Deng, J.; Zhu, Y. Thrombus Enhancement Sign May Differentiate Embolism From Arteriosclerosis-Related Large Vessel Occlusion. J. Stroke 2023, 25, 233–241. [Google Scholar] [CrossRef]
- Ayad, M.; Eskioglu, E.; Mericle, R.A. Onyx®: A unique neuroembolic agent. Expert Rev. Med. Devices 2006, 3, 705–715. [Google Scholar] [CrossRef]
- Carberry, G.; Dalvie, P.; Ozkan, O. Onyx as a second-line embolic agent in peripheral applications. J. Vasc. Interv. Radiol. 2013, 24, S103. [Google Scholar] [CrossRef]
- Pop, R.; Mertz, L.; Ilyes, A.; Mihoc, D.; Richter, J.S.; Manisor, M.; Kremer, S.; Beaujeux, R. Beam hardening artifacts of liquid embolic agents: Comparison between Squid and Onyx. J. NeuroInt. Surg. 2019, 11, 706–709. [Google Scholar] [CrossRef]
- Venturini, M.; Piacentino, F.; Coppola, A.; Fontana, F. Editorial of Special Issue “Embolization Techniques: State of the Art and Future Perspectives”. J. Clin. Med. 2022, 11, 5109. [Google Scholar] [CrossRef]
- Petrov, A.; Ivanov, A.; Kolomin, E.; Tukanov, N.; Petrova, A.; Rozhchenko, L.; Suvorova, J. The Advantages of Non-Adhesive Gel-like Embolic Materials in the Endovascular Treatment of Benign Hypervascularized Lesions of the Head and Neck. Gels 2023, 9, 954. [Google Scholar] [CrossRef]
- Kuianova, I.; Chupakhin, A.; Besov, A.; Gorbatykh, A.; Kislitsin, D.; Orlov, K.; Parshin, D. Rheological Properties of Non-Adhesive Embolizing Compounds—The Key to Fine-Tuning Embolization Process-Modeling in Endovascular Surgery. Polymers 2023, 15, 1060. [Google Scholar] [CrossRef]
- Vollherbst, D.F.; Chapot, R.; Bendszus, M.; Möhlenbruch, M.A. Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas. Clin. Neuroradiol. 2022, 32, 25–38. [Google Scholar] [CrossRef]
- Dhar, S.; Lee, A.; Chaskes, M.B.; Dehdashti, A.R.; Elijovich, L.; Michael, M.; Patsalides, A.; Rangarajan, S.V.; Fastenberg, J.H. Onyx or Glue? A Representative Case Comparison of Embolic Agents for Direct Puncture Embolization of Juvenile Nasopharyngeal Angiofibroma. J. Neurol. Surg. B Skull Base 2024, 85, S1–S398. [Google Scholar] [CrossRef]
- Guan, J.J.; Golzarian, J. Review of Commonly Used Embolics. Dig. Dis. Interv. 2024, 8. [Google Scholar] [CrossRef]
- Wang, W.; Lin, E.; Zhang, D.; Crane, B. Onyx Embolization Material Extrusion in the Middle Ear. Otol. Neurotol. 2019, 40, e847–e849. [Google Scholar] [CrossRef]
- Xiao, N.; Lewandowski, R.J. Embolic Agents: Coils. Semin Interv. Radiol. 2022, 39, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Frandon, J.; Loffroy, R.; Marcelin, C.; Vernhet-Kovacsik, H.; Greffier, J.; Dabli, D.; Sammoud, S.; Marek, P.; Chevallier, O.; Beregi, J.-P.; et al. Safety and Efficacy of Prestige Coils for Embolization of Vascular Abnormalities: The Embo-Prestige Study. J. Pers. Med. 2023, 13, 1464. [Google Scholar] [CrossRef]
- Roh, H.; Kim, J.; Bae, H.; Chong, K.; Kim, J.H.; Suh, S.; Kwon, T.; Yoon, W. Comparison of stent-assisted and no-stent coil embolization for safety and effectiveness in the treatment of ruptured intracranial aneurysms. J. Neurosurg. JNS 2020, 133, 814–820. [Google Scholar] [CrossRef]
- Xu, H.; Jing, C.; Zhou, J.; Min, X.; Zhao, J.; Yang, L.; Ren, Y. Clinical efficacy of coil embolization in treating pseudoaneurysm post-Whipple operation. Exp. Ther. Med. 2020, 20, 37. [Google Scholar] [CrossRef]
- Fatemi, N.; Lee, A.; Kessler, J.; Fang, J.; Park, J.M.; Park, J.J. Coil or Plug-Assisted Ethylene Vinyl Alcohol Copolymer (EVOH) Thoracic Duct Embolization in the Treatment of Postoperative Chylothorax. J. Vasc. Interv. Radiol. 2024, 35, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Bagchee-Clark, A.; Martin, J. Embolization Materials and Principles. In Demystifying Interventional Radiology; Athreya, S., Albahhar, M., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Tyfa, Z.; Tomasik, B.; Reorowicz, P.; Bobeff, E.J.; Posmyk, B.J.; Hupało, M.; Stefańczyk, L.; Jóźwik, K.; Jaskólski, D.J. Risk Factors for Recanalization after Coil Embolization. J. Pers. Med. 2021, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Abi-Aad, K.R.; Rahme, R.J.; Patra, D.P.; Turcotte, E.L.; Richter, K.R.; Merrill, S.A.; Syal, A.; Neville, M.R.; Hudson, M.; Garcia, J.O.; et al. Clinical outcomes of first- and second-generation hydrogel coils compared with bare platinum coils: A systematic literature review. Neurosurg. Rev. 2022, 45, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Cao, L.; Ding, Y.; Han, Y.; Yu, X.; Cui, J.; Wang, H.; Wu, J.; El-Newehy, M.; Abdulhameed, M.M.; et al. Modified Highly Elastic 3D Nanofiber Embolic Scaffolds for Precise In Situ Embolization Therapy. Adv. Funct. Mater. 2024, 2316590. [Google Scholar] [CrossRef]
- Albadawi, H.; Altun, I.; Hu, J.; Zhang, Z.; Panda, A.; Kim, H.; Khademhosseini, A.; Oklu, R. Nanocomposite Hydrogel with Tantalum Microparticles for Rapid Endovascular Hemostasis. Adv. Sci. 2021, 8, 2003327. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-based hydrogels for tissue engineering applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Albadawi, H.; Zhang, Z.; Salomao, M.A.; Gunduz, S.; Rehman, S.; D’Amone, L.; Mayer, J.L.; Omenetto, F.; Oklu, R. Silk embolic material for catheter-directed endovascular drug delivery. Adv. Mater. 2022, 34, 2106865. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Shen, M.; Huang, L.; Ding, L.; Hu, J.; Dong, Y.; Fu, H.; Wang, Q.; Sun, Y.; et al. Precision Embolism: Biocompatible Temperature-Sensitive Hydrogels as Novel Embolic Materials for Both Mainstream and Peripheral Vessels. Adv. Funct. Mater. 2021, 31, 2011170. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Bonani, W.; Reis, R.L.; Migliaresi, C.; Ferreira, H.; Motta, A.; Neves, N.M. Development of alginate-based hydrogels for blood vessel engineering. Biomater. Adv. 2022, 134, 112558. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Mazzotta, E.; Marazioti, A.; Mourtas, S.; Muzzalupo, R.; Antimisiaris, S.G. Liposomes Coated with Novel Synthetic Bifunctional Chitosan Derivatives as Potential Carriers of Anticancer Drugs. Pharmaceutics 2024, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Zehtabi, F.; Lerouge, S. Optimization and characterization of injectable chitosan-iodixanol-based hydrogels for the embolization of blood vessels. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.; Lu, Z.; Wang, X.; Bai, S.; Chen, Y.; Mao, J.; Liu, G. Liquid embolic agents for interventional embolization. Chem. Phys. Mater. 2022, 1, 39–50. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; Luo, Q.; Li, Y.; Sun, L.; Wang, H.; Xu, K.; Wang, B.; Zhen, Y. In vivo assessment of chitosan/beta-glycerophosphate as a new liquid embolic agent. Interv. Neuroradiol. 2011, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Chabrot, P.; Berrahmoune, S.; Coutu, J.-M.; Soulez, G.; Lerouge, S. A new injectable radiopaque chitosan-based sclerosing embolizing hydrogel for endovascular therapies. Acta Biomater. 2012, 8, 2712–2721. [Google Scholar] [CrossRef]
- Shili, A.; Qinzong, G.; Gele, C.; Pengfei, Z.; Peiyu, C.; Yingying, R.; Hao, W.; Xu, Z.; Shanyue, G.; Xiaozhong, Q. Construction of an Injectable Composite Double-Network Hydrogel as a Liquid Embolic Agent. Biomacromolecules 2024, 25, 2052–2064. [Google Scholar] [CrossRef]
- Ning, X.; Zhao, C.; Zhao, J.; Pang, J. Evaluating thermosensitive chitosan/ β-glycerophosphate sodium and fibroblast embolization for the treatment of cerebral arteriovenous malformation in a porcine model. Neuroendocrinol. Lett. 2022, 43, 385–392. [Google Scholar] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.; Jiang, Z. Alginate oligosaccharides: Production, biological activities, and potential applications. J. Compr. Rev. Food Sci. Food Saf. 2021, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of alginate-based biomaterials and their applications in biomedicine. J. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Maity, C.; Das, N. Alginate-based smart materials and their application: Recent advances and perspectives. J. Top. Curr. Chem. 2022, 380, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Duan, M.; Xie, Z.; Pan, K.; Wang, X.; Sun, X.; Wang, Q.; Rao, W.; Liu, J. Injectable and Radiopaque Liquid Metal/Calcium Alginate Hydrogels for Endovascular Embolization and Tumor Embolotherapy. Small 2020, 16, e1903421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, M.; Su, X.; Yuan, W.; Feng, W.; Su, Q.; Li, F. Afterglow implant for arterial embolization and intraoperative imaging. J. Chem. Eur. J. 2022, 28, e202103795. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Chen, Y.-C.; Zhao, Y.; Yodsanit, N.; Wang, Y.; Yamamoto, N.; Yamanouchi, D.; Gong, S. Injectable Hydrogel Capable of In Situ Covalent Crosslinking for Permanent Embolization. ACS Appl. Mater. Interfaces 2021, 13, 56988–56999. [Google Scholar] [CrossRef]
- Griswold, E.; Cappello, J.; Ghandehari, H. Silk-elastinlike protein-based hydrogels for drug delivery and embolization. Adv. Drug Deliv. Rev. 2022, 191, 114579. [Google Scholar] [CrossRef]
- Cipriani, F.; Krüger, M.; de Torre, I.G.; Sierra, L.Q.; Rodrigo, M.A.; Kock, L.; Rodriguez-Cabello, J.C. Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Steinhauff, D.; Jensen, M.; Talbot, M.; Jia, W.; Isaacson, K.; Jedrzkiewicz, J.; Cappello, J.; Oottamasathien, S.; Ghandehari, H. Silk-elastinlike copolymers enhance bioaccumulation of semisynthetic glycosaminoglycan ethers for prevention of radiation induced proctitis. J. Control. Release 2021, 332, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Steinhauff, D.; Jensen, M.M.; Griswold, E.; Jedrzkiewicz, J.; Cappello, J.; Oottamasathien, S.; Ghandehari, H. An Oligomeric Sulfated Hyaluronan and Silk-Elastinlike Polymer Combination Protects against Murine Radiation Induced Proctitis. Pharmaceutics 2022, 14, 175. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Jensen, M.; Steinhauff, D.; Wei, X.; Huo, E.; Jedrzkiewicz, J.; Cappello, J.; Cheney, D.; Ghandehari, H. Translational Development of a Silk-Elastinlike Protein Polymer Embolic for Transcatheter Arterial Embolization. Macromol. Biosci. 2022, 22, e2100401. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.M.; Hatlevik, Ø.; Steinhauff, D.D.; Griswold, E.D.; Wei, X.; Isaacson, K.J.; Barber, Z.B.; Huo, E.; Taussky, P.; Jedrzkiewicz, J.; et al. Protein-based polymer liquid embolics for cerebral aneurysms. Acta Biomater. 2022, 151, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Dai, C.-Y.; Mao, X.; Lv, X.; Gu, Y.; Lee, E.-S.; Jiang, H.-B.; Sun, Y. Poloxamer-Based Scaffolds for Tissue Engineering Applications: A Review. Gels 2022, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef] [PubMed]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Qassemyar, Q.; Michel, G.; Gianfermi, M.; Atlan, M.; Havet, E.; Luca-Pozner, V. Sutureless venous microanastomosis using thermosensitive poloxamer and cyanoacrylate: Experimental study on a rat model. J. Plast. Reconstr. Aesthetic Surg. 2021, 75, 433–438. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, D.; Ren, Y.; Li, L.; Zhao, Y.; Zheng, C.; Yang, X. The immune-chemo-embolization effect of temperature sensitive gold nanomedicines against liver cancer. Nano Res. 2023, 16, 2749–2761. [Google Scholar] [CrossRef]
- Traci, M.; Roy, R.; Upchurch, G.J. Aortoiliacaneurysms: Evaluation, decision, making, and medical management. In Rutherford’s VascularSurgery and EndovascularTherapy, 9th ed.; Sidawy, A., Perler, B., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 884–893. [Google Scholar]
- Wei, L.; Bu, X.; Wang, X.; Liu, J.; Ma, A.; Wang, T. Global burden of aortic aneurysm and attributable risk factors from 1990 to 2017. Glob. Heart 2021, 16, 35. [Google Scholar] [CrossRef]
- Kapila, V.; Jetty, P.; Wooster, D.; Vucemilo, V.; Dubois, L.; Canadian Society for Vascular Surgery. Screening for abdominal aortic aneurysms in Canada: 2020 review and position statement of the Canadian Society for Vascular Surgery. Can. J. Surg. 2021, 64, E461–E466. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.M.; Rafferty, E.A.; Geller, S.C.; Kaufman, J.A.; Brewster, D.C.; Cambria, R.P.; Waltman, A.C. Endovascular sten graft in abdominal aortic aneurysms:the relationship between patent vessels that arise from the aneurysmal sac and early endoleak. Radiology 2001, 218, 176–182. [Google Scholar] [CrossRef]
- van Schaik, T.G.; Meekel, J.P.; Hoksbergen, A.W.; de Vries, R.; Blankensteijn, J.D.; Yeung, K.K. Systematic review of embolization of type I endoleaks using liquid embolic agents. J. Vasc. Surg. 2021, 74, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ameli-Renani, S.; Pavlidis, V.; Morgan, R.A. Early and midterm outcomes after transcatheter embolization of type I endoleaks in 25 patients. J. Vasc. Surg. 2017, 65, 346–355. [Google Scholar] [CrossRef] [PubMed]
- van Marrewijk, C.; Buth, J.; Harris, P.L.; Norgren, L.; Nevelsteen, A.; Wyatt, M.G. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: The EUROSTAR experience. J. Vasc. Surg. 2002, 35, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.-Y.; Morgan, R. Transcatheter Embolisation of Type 1 Endoleaks after Endovascular Aortic Aneurysm Repair with Onyx: When No Other Treatment Option is Feasible. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Barburoglu, M.; Sencer, S.; Berdikhojayev, M.; Coskun, B.; Akpek, S. Flow Diversion with Low-Profile Braided Stents for the Treatment of Very Small or Uncoilable Intracranial Aneurysms at or Distal to the Circle of Willis. AJNR Am. J. Neuroradiol. 2017, 38, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Mozes, G.D.; Pather, K.; Oderich, G.S.; Mirza, A.; Colglazier, J.J.; Shuja, F.; Mendes, B.C.; Kalra, M.; Bjarnason, H.; Bower, T.C.; et al. Outcomes of Onyx® Embolization of Type II Endoleaks After Endovascular Repair of Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2020, 67, 223–231. [Google Scholar] [CrossRef]
- Scallan, O.; Kribs, S.; Power, A.H.; DeRose, G.; Duncan, A.; Dubois, L. Onyx versus coil embolization for the treatment of type II endoleaks. J. Vasc. Surg. 2021, 73, 1966–1972. [Google Scholar] [CrossRef]
- Barnett, B.P.; Gailloud, P. Assessment of EmboGel—A Selectively Dissolvable Radiopaque Hydrogel for Embolic Applications. J. Vasc. Interv. Radiol. JVIR 2011, 22, 203–211. [Google Scholar] [CrossRef]
- Sivakumaran, L.; Alturkistani, H.; Lerouge, S.; Bertrand-Grenier, A.; Zehtabi, F.; Thérasse, É.; Roy-Cardinal, M.-H.; Bhatnagar, S.; Cloutier, G.; Soulez, G. Strain Ultrasound Elastography of Aneurysm Sac Content after Randomized Endoleak Embolization with Sclerosing vs. Non-sclerosing Chitosan-based Hydrogels in a Canine Model. J. Vasc. Interv. Radiol. 2022, 33, 495–504.e3. [Google Scholar] [CrossRef]
- Schimmel, K.; Ali, M.K.; Tan, S.Y.; Teng, J.; Do, H.M.; Steinberg, G.K.; Stevenson, D.A.; Spiekerkoetter, E. Arteriovenous Malformations—Current Understanding of the Pathogenesis with Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 9037. [Google Scholar] [CrossRef]
- Dunham, G.M.; Ingraham, C.R.; Maki, J.H.; Vaidya, S.S. Finding the Nidus: Detection and Workup of Non-Central NervousSystem Arteriovenous Malformations. Radiographics 2016, 36, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Legiehn, G.M.; Heran, M.K.S. Classification, Diagnosis, and Interventional Radiologic Management of Vascular Malformations. Orthop. Clin. N. Am. 2006, 37, 435–474. [Google Scholar] [CrossRef]
- Mulligan, P.R.; Prajapati, H.J.S.; Martin, L.G.; Patel, T.H. Vascular anomalies: Classification, imaging characteristics and implications for interventional radiology treatment approaches. Br. J. Radiol. 2014, 87, 20130392. [Google Scholar] [CrossRef] [PubMed]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.X.; Oh, S.S.; McWilliams, J.P. Bronchoscopy-guided removal of intrabronchial coil migration after coil embolization of pulmonary arteriovenous malformation. Radiol. Case Rep. 2022, 17, 3410–3414. [Google Scholar] [CrossRef]
- Shimohira, M.; Kawai, T.; Hashizume, T.; Muto, M.; Kitase, M.; Shibamoto, Y. Usefulness of Hydrogel-Coated Coils in Embolization of Pulmonary Arteriovenous Malformations. Cardiovasc. Interv. Radiol. 2018, 41, 848–855. [Google Scholar] [CrossRef]
- Iguchi, T.; Hiraki, T.; Matsui, Y.; Fujiwara, H.; Sakurai, J.; Baba, K.; Toyooka, S.; Gobara, H.; Kanazawa, S. Embolization using hydrogel-coated coils for pulmonary arteriovenous malformations. Diagn. Interv. Imaging 2020, 101, 129–135. [Google Scholar] [CrossRef]
- Velinov, N.; Petrov, M.; Sakelarova, T.; Yordanov, P.; Martinov, I.; Gabrovsky, N. Endovascular treatment with Onyx of arterio-venous fistula around the transverse sinus presenting with ocular symptoms. Case report. Interdiscip. Neurosurg. 2023, 36, 101932. [Google Scholar] [CrossRef]
- Rajab, I.M.; Adas, A.; Shubietah, A.R.; Khader, M.I. Successful embolization of an anterior chest wall arteriovenous malformation using combined transfemoral and transradial approaches with onyx. Radiol. Case Rep. 2024, 19, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.C.; Dobashi, Y.; Pasarikovski, C.R.; Ramjist, J.; Madden, J.D.; Walus, K.; Yang, V.X. Dynamic laser-based photomodulation of endovascular hydrogel embolization for the treatment of various cerebrovascular disorders. In Clinical and Translational Neurophotonics; SPIE: Yokohama, Japan, 2022; Volume 11945, p. 1194503. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, P.; Sena, G.; Piro, P.; De Bartolo, T.; Galassi, S.; Costa, D.; Serra, R. OnyxTMGel or Coil versus Hydrogel as Embolic Agents in Endovascular Applications: Review of the Literature and Case Series. Gels 2024, 10, 312. https://doi.org/10.3390/gels10050312
Perri P, Sena G, Piro P, De Bartolo T, Galassi S, Costa D, Serra R. OnyxTMGel or Coil versus Hydrogel as Embolic Agents in Endovascular Applications: Review of the Literature and Case Series. Gels. 2024; 10(5):312. https://doi.org/10.3390/gels10050312
Chicago/Turabian StylePerri, Paolo, Giuseppe Sena, Paolo Piro, Tommaso De Bartolo, Stefania Galassi, Davide Costa, and Raffaele Serra. 2024. "OnyxTMGel or Coil versus Hydrogel as Embolic Agents in Endovascular Applications: Review of the Literature and Case Series" Gels 10, no. 5: 312. https://doi.org/10.3390/gels10050312