Growth of Floc Structure and Subsequence Compaction into Smaller Granules through Breakup and Rearrangement of Aluminum Flocs in a Constant Laminar Shear Flow
Abstract
:1. Introduction
2. Results and Discussion
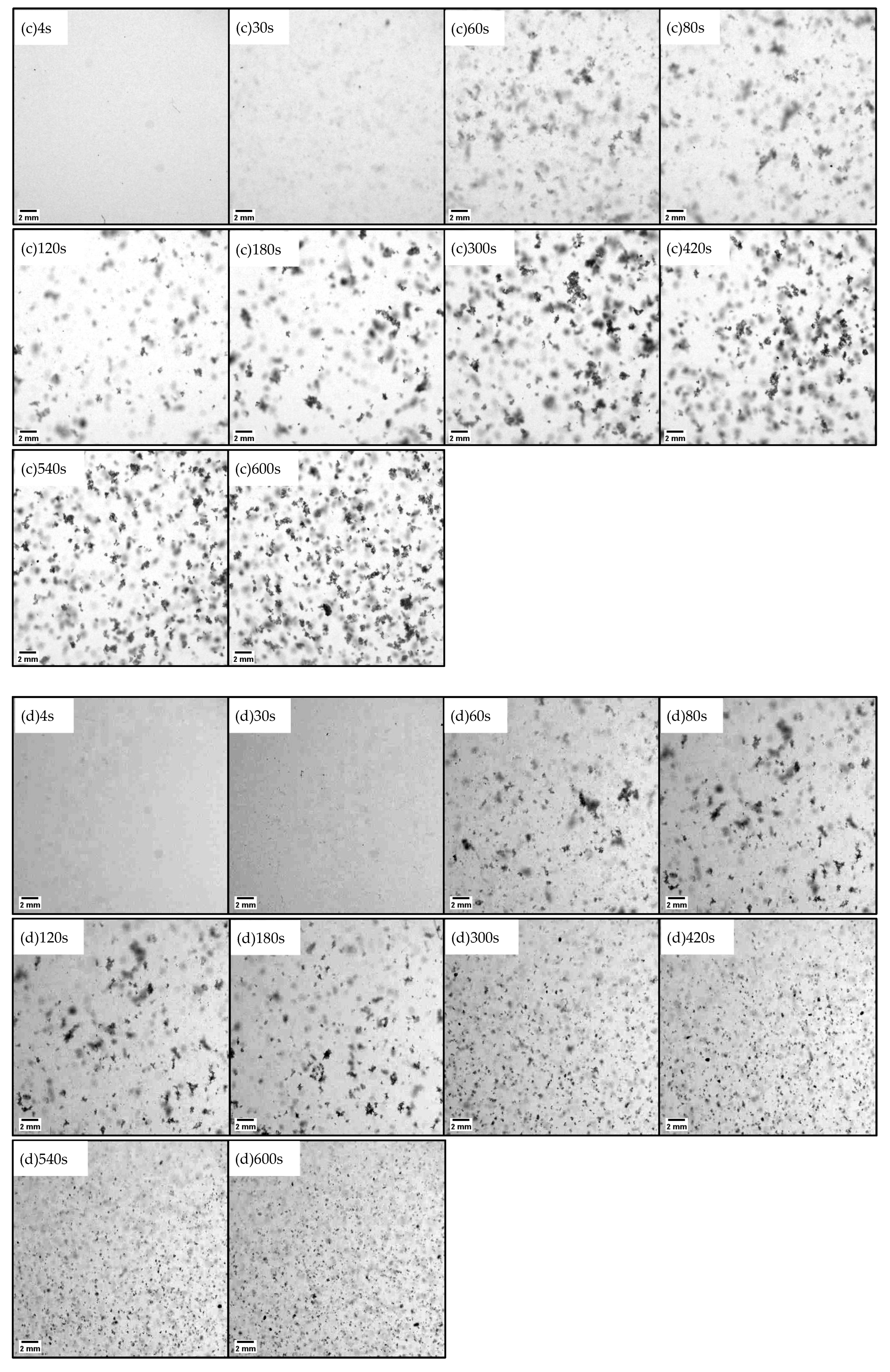
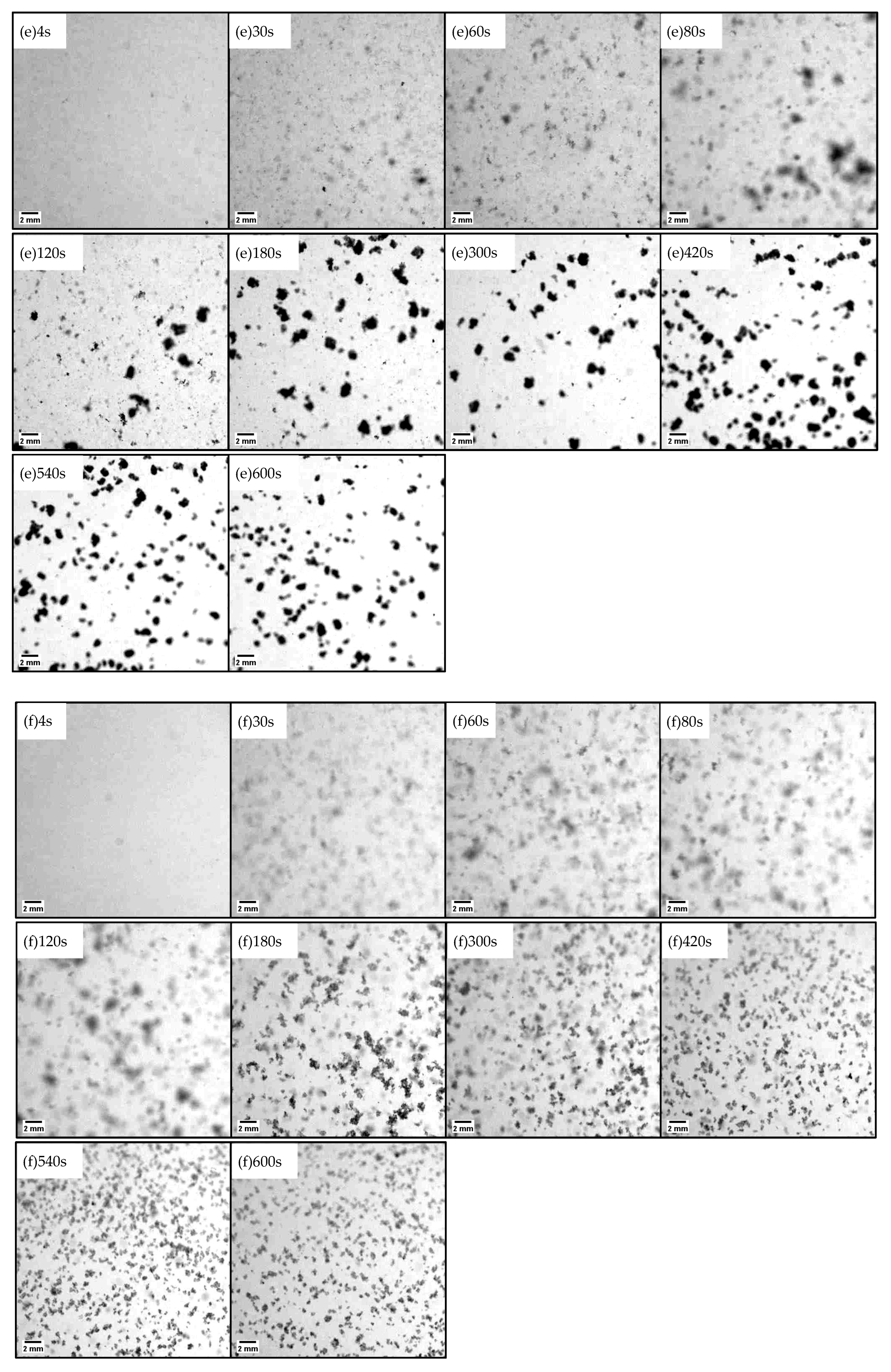
2.1. Floc Images and Structual Change
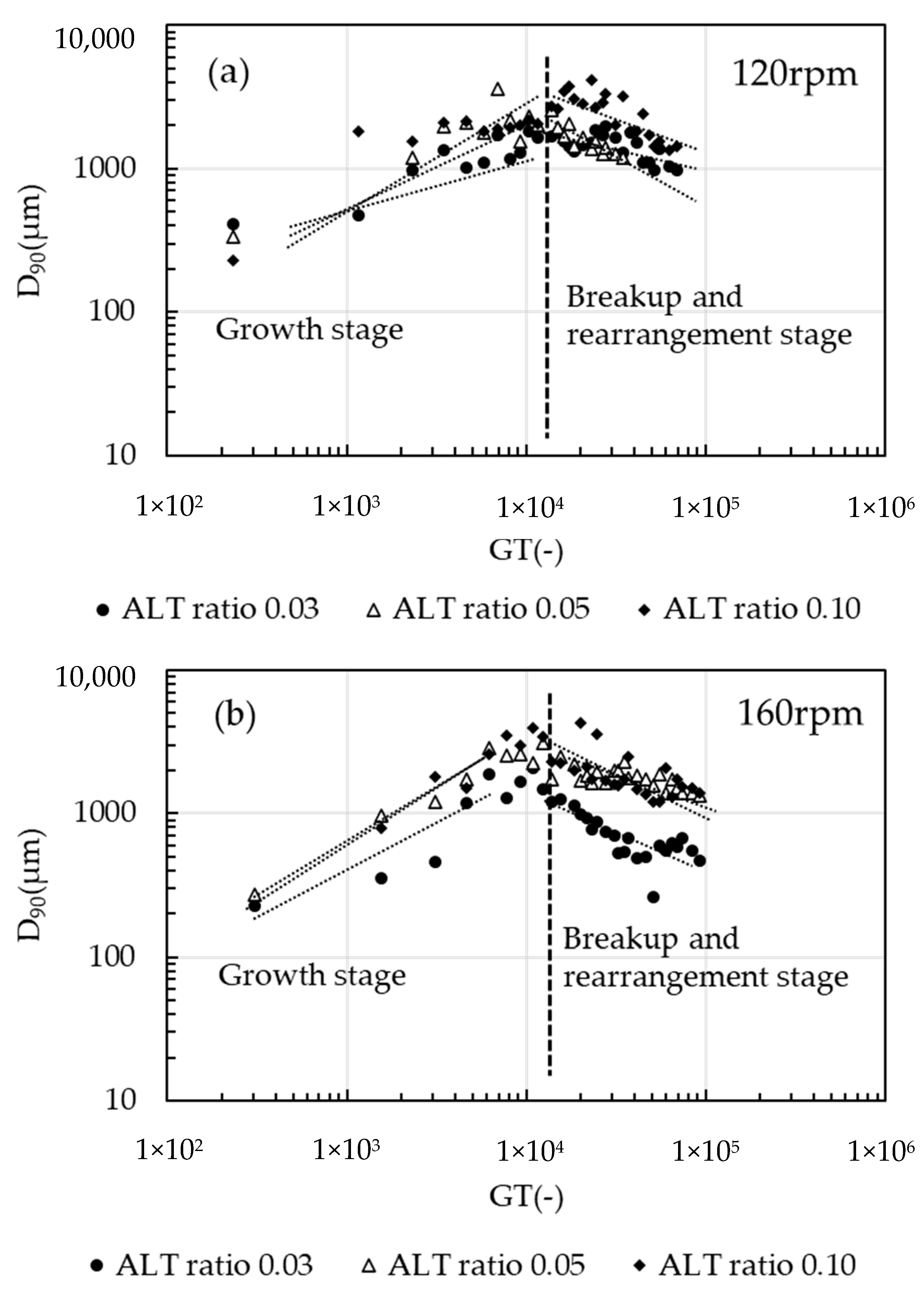
2.2. Floc Growth and Breakup/Rearrengement Stages
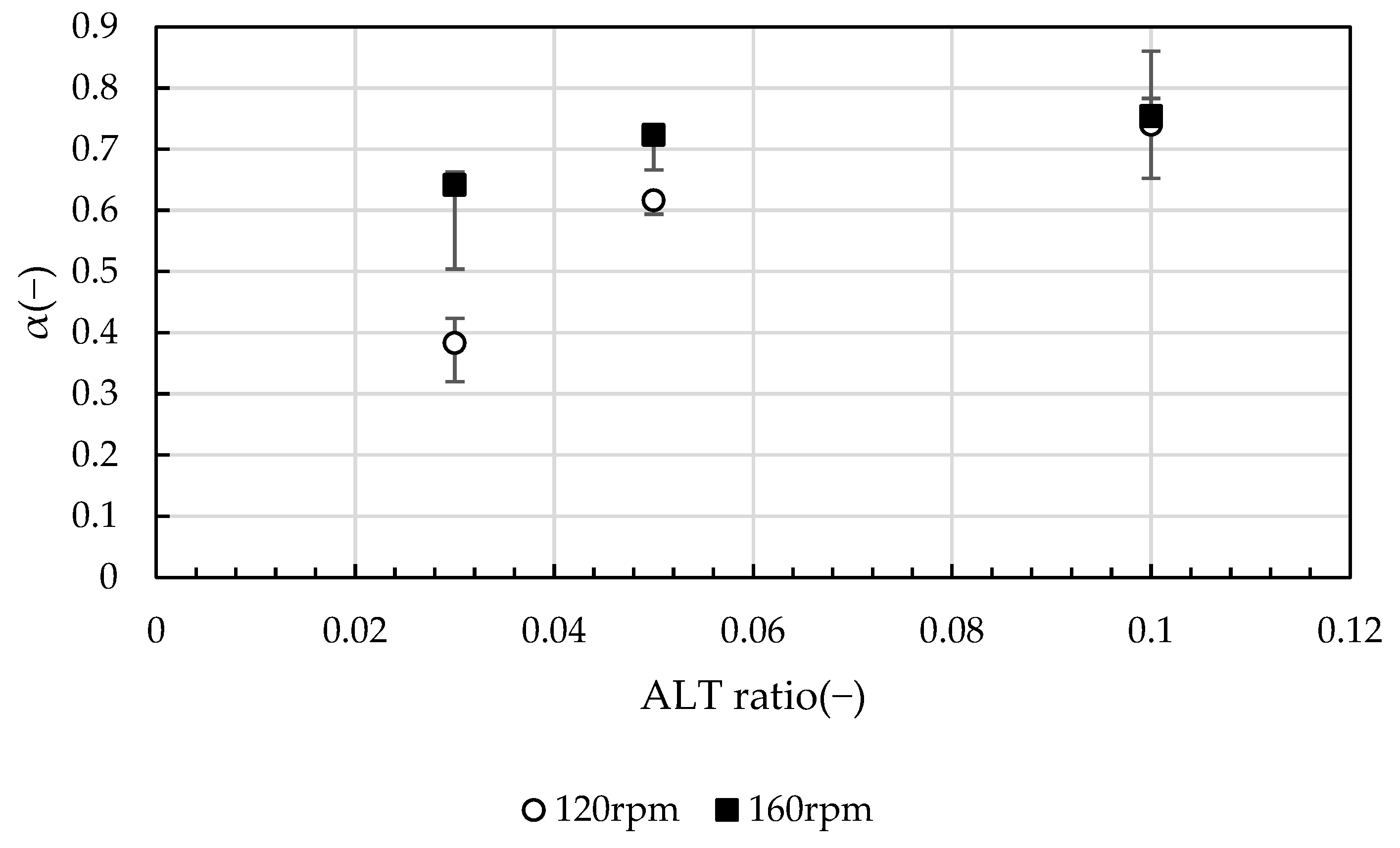
2.2.1. Evaluation of Growth Stage
2.2.2. Evaluation of Breakup/Rearrangement Stage
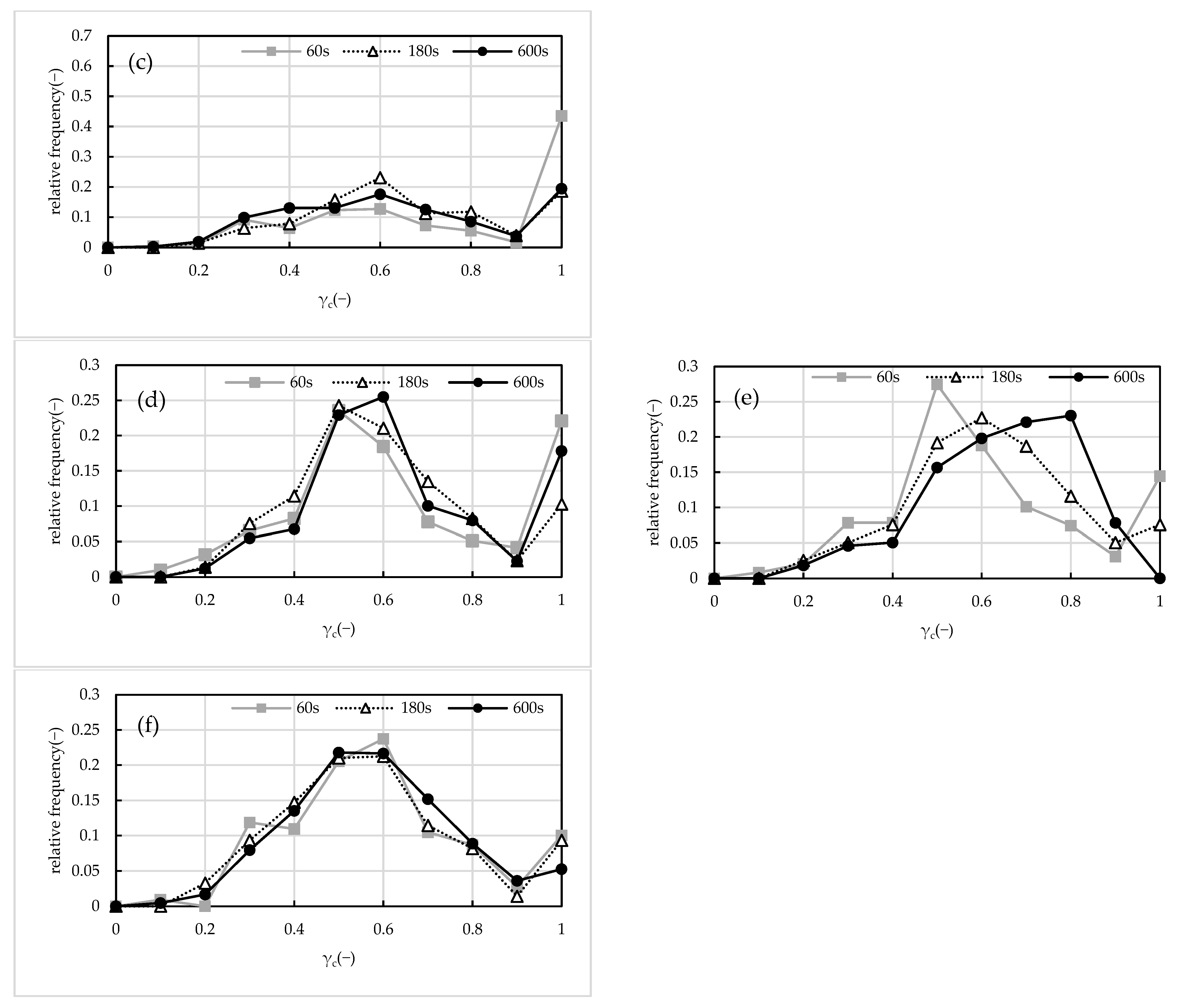
2.3. Floc Circularity
2.4. Compactification of Floc Structure
3. Conclusions
- After the floc structure develops and becomes bulky, the floc becomes fine through breakup and rearrangement;
- The growth rate and breakup/rearrangement rate have a power-law relationship with respect to the GT value. Additionally, growth and breakup/rearrangement stages are clearly distinguishable around a certain GT value;
- Aggregation rate α during the growth stage increases with the ALT ratio. In an ALT ratio of 0.10, the effect of the G value is less significant;
- In the breakup/rearrangement stage, at ALT ratios of 0.30 and 0.50, β depends more on G values than on ALT ratios; at the ALT ratio of 0.10, β depends more on the ALT ratio than on G values;
- In the breakup/rearrangement stage, the floc diameter is maintained with respect to the G value under near-charge-neutralization conditions (an ALT ratio 0.05). Separate breakup is followed by erosive breakup, and the flocs become denser by incorporating the small fragments generated. Erosion breakup predominates and the floc granulates spherically. The granulation of flocs is related to the gelation of aluminum hydroxide gel.
4. Materials and Methods
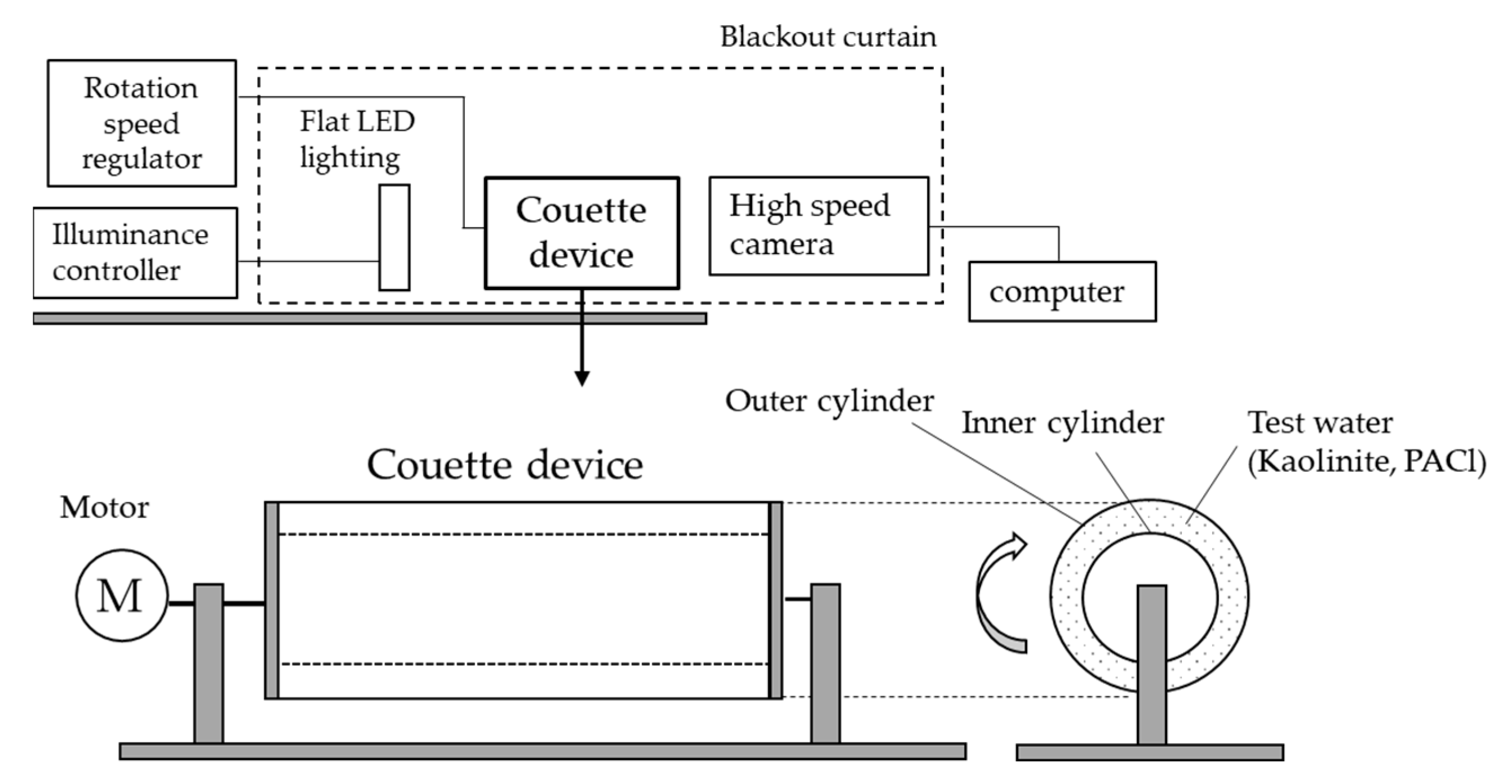
4.1. Couette Device and Photographic Equipment
4.2. Image Analysis and Data Statistics
4.3. Test Water Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bache, D.H.; Gregory, R. Flocs in Water Treatment; IWA Publishing: London, UK, 2007; Volume 6, ISBN 9781780402000. [Google Scholar] [CrossRef]
- Smoluchowski, M. Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Z. Für Phys. Chem. 1918, 92, 129–168. [Google Scholar] [CrossRef]
- Tambo, N.; Watanabe, Y. Physical characteristics of flocs–I. The floc density function and aluminum floc. Water Res. 1979, 13, 409–419. [Google Scholar] [CrossRef]
- Tambo, N.; Watanabe, Y. A study on aluminum floc density–I. J. Jpn. Water Work. Assoc. 1967, 397, 2–10. [Google Scholar]
- Kusuda, T. Effects on floc properties by condition of floc formation. Proc. Jpn. Soc. Civ. Eng. 1973, 217, 33–39. [Google Scholar] [CrossRef]
- Sutherland, D.N.; Goodarz-Nia, I. Floc simulation: The effect of collision sequence. Chem. Eng. Sci. 1971, 26, 2071–2085. [Google Scholar] [CrossRef]
- Meakin, P.; Jullian, R. The effects of restructuring on the geometry of clusters formed by diffusion-limited, ballistic, and reaction-limited cluster–cluster aggregation. J. Chem. Phys. 1988, 89, 246–250. [Google Scholar] [CrossRef]
- Meakin, P. Fractal Aggregates. Adv. Colloid Interface Sci. 1987, 28, 249–331. [Google Scholar] [CrossRef]
- Adachi, Y.; Ooi, S. Geometrical structure of a floc. J. Colloid Interface Sci. 1990, 135, 374–384. [Google Scholar] [CrossRef]
- Weitz, D.A.; Huang, J.S.; Lin, M.Y.; Sung, J. Limits of the fractal dimension for irreversible kinetic aggregation of gold colloids. Phys. Rev. Lett. 1985, 54, 1416–1419. [Google Scholar] [CrossRef]
- Aubert, C.; Cannell, D.S. Restructuring of colloidal silica aggregates. Phys. Rev. Lett. 1986, 56, 738–741. [Google Scholar] [CrossRef]
- Oles, V. Shear-induced aggregation and breakup of polystyrene latex particles. J. Colloid Interface Sci. 1992, 154, 351–358. [Google Scholar] [CrossRef]
- Gregory, J. The density of particle aggregates. Water Sci. Technol. 1997, 36, 1–13. [Google Scholar] [CrossRef]
- Tambo, N.; Hozumi, H. Physical characteristics of flocs–II. Strength of floc. Water Res. 1979, 13, 421–427. [Google Scholar] [CrossRef]
- Kobayashi, M.; Adachi, Y.; Ooi, S. Breakup of fractal flocs in a turbulent flow. Langmuir 1999, 15, 4351–4356. [Google Scholar] [CrossRef]
- Chen, D.; Doi, M. Simulation of Aggregating Colloids in Shear Flow. II. J. Chem. Phys. 1989, 91, 2656–2663. [Google Scholar] [CrossRef]
- Higashitani, K.; Iimura, K.; Sanda, H. Simulation of deformation and breakup of large aggregates in flows of viscous fluids. Chem. Eng. Sci. 2001, 56, 2927–2938. [Google Scholar] [CrossRef]
- Jarvis, P.; Jefferson, B.; Gregory, J.; Parsons, S.A. A review of floc strength and breakage. Water Res. 2005, 39, 3121–3137. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Kobayashi, M.; Yanagibashi, T. Densification of small flocs formed in the mixing flow under the condition of rapid coagulation. Trans. Jpn. Soc. Irrig. Drain. Reclam. Eng. 2004, 233, 497–504. [Google Scholar] [CrossRef]
- Spicer, P.T.; Keller, W.; Pratsinis, S.E. The effect of impeller type on floc size and structure during shear-induced flocculation. J. Colloid Interface Sci. 1996, 184, 112–122. [Google Scholar] [CrossRef]
- Soos, M.; Moussa, A.S.; Ehrl, L.; Sefcik, J.; Wu, H.; Morbidelli, M. Effect of shear rate on aggregate size and morphology investigated under turbulent conditions in stirred tank. J. Colloid Interface Sci. 2008, 319, 577–589. [Google Scholar] [CrossRef]
- Yeung, A.K.C.; Pelton, R. Micromechanics: A new approach to studying the strength and breakup of flocs. J. Colloid Interface Sci. 1996, 184, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Blaser, S. Break-up of flocs in contraction and swirling flows. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 215–223. [Google Scholar] [CrossRef]
- Blaser, S. Flocs in shear and strain flows. J. Colloid Interface Sci. 2000, 225, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Strength of natural soil flocs. Water Res. 2005, 39, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Kobayashi, M.; Fukuhara, Y. Break-up strength of flocs analyzed using orifice converging flow. Nihon Reoroji Gakkaishi 2007, 35, 69–72. [Google Scholar] [CrossRef]
- Sonntag, R.C.; Russel, W.B. Structure and breakup of flocs subjected to fluid stresses: I. Shear experiments. J. Colloid Interface Sci. 1986, 113, 399–413. [Google Scholar] [CrossRef]
- Frappier, G.; Lartiges, B.S.; Skali-Lami, S. Floc cohesive force in reversible aggregation: A couette laminar flow investigation. Langmuir 2010, 26, 10475–10488. [Google Scholar] [CrossRef]
- Serra, T.; Casamitjana, X. Structure of the aggregates during the process of aggregation and breakup under a shear flow. J. Colloid Interface Sci. 1998, 206, 505–511. [Google Scholar] [CrossRef]
- Selomulya, C.; Amal, R.; Bushell, G.; Waite, T.D. Evidence of shear rate dependence on restructuring and breakup of latex aggregates. J. Colloid Interface Sci. 2001, 236, 67–77. [Google Scholar] [CrossRef]
- Selomulya, C.; Amal, R.; Bushell, G.; Waite, T.D. Aggregation Mechanisms of Latex of Different Particle Sizes in a Controlled Shear Environment. Laminar 2002, 18, 1974–1984. [Google Scholar] [CrossRef]
- Selomulya, B.C.; Amal, R.G.; Waite, T.D. Understanding of the role of restructuring in flocculation: The application of a population balance model. J. Colloid Interface Sci. 2003, 58, 327–328. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Concha, F.; Toledo, P.G. Population balance modelling of particle flocculation with attention to aggregate restructuring and permeability. Adv. Colloid Interface Sci. 2015, 224, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Hyunseop, L.; Chongyoup, K. Experimental study on reversible formation of 2D flocs from plate-like particles dispersed in Newtonian fluid under torsional flow. Colloids Surf. A Physicochem. Eng. Asp. 2018, 548, 70–84. [Google Scholar]
- Guérin, L.; Coufort-Saudejaud, C.; Liné, A.; Christ, F. Dynamics of aggregate size and shape properties under sequenced flocculation in a turbulent Taylor-Couette reactor. J. Colloid Interface Sci. 2017, 491, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hayami, M.F.; Menju, T.; Ide, T.; Uchida, T.; Sakurai, S.; Adachi, Y. Structural change of poly-aluminum chloride and kaolinite floc in constant shear flow by the Couette device. J. EICA 2023, 27, 82–90. [Google Scholar]
- Adachi, Y.; Tanaka, Y.; Ooi, S. The structure of a kaolinite floc coagulated with aluminum salt. Trans. Jpn. Soc. Irrig. Drain. Reclam. Eng. 1999, 203, 593–599. [Google Scholar] [CrossRef]
- Chigier, N. Optical imaging of sprays. Prog. Energy Combust. Sci. 1991, 17, 211–262. [Google Scholar] [CrossRef]
- Chigier, N. Drop size and velocity instrumentation. Prog. Energy Combust. Sci. 1983, 9, 155–177. [Google Scholar] [CrossRef]
- Ow, C.S.; Crane, R.I. A simple off-line automatic image analysis system with application to drop sizing in two-phase flows. Int. J. Heat Fluid Flow 1980, 2, 47–53. [Google Scholar] [CrossRef]
- Adachi, Y.; Di, C.; Xiao, F.; Kobayashi, M. Size, orientation, and strength of Na-montmorillonite flocs flowing in a laminar shear flow. Colloid Polym. Sci. 2019, 297, 979–987. [Google Scholar] [CrossRef]
- Ferreira, T.; Rasband, W. ImageJ User Guide IJ 1.46r. Available online: https://imagej.nih.gov/ij/docs/guide/user-guide.pdf (accessed on 2 October 2012).
- Ushiyama, T. How to use the waste water treatment chemicals. Jpn. TAPPI J. 2012, 66, 29–35. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayami, M.F.; Menju, T.; Ide, T.; Uchida, T.; Adachi, Y. Growth of Floc Structure and Subsequence Compaction into Smaller Granules through Breakup and Rearrangement of Aluminum Flocs in a Constant Laminar Shear Flow. Gels 2024, 10, 49. https://doi.org/10.3390/gels10010049
Hayami MF, Menju T, Ide T, Uchida T, Adachi Y. Growth of Floc Structure and Subsequence Compaction into Smaller Granules through Breakup and Rearrangement of Aluminum Flocs in a Constant Laminar Shear Flow. Gels. 2024; 10(1):49. https://doi.org/10.3390/gels10010049
Chicago/Turabian StyleHayami, Mii Fukuda, Takashi Menju, Takeshi Ide, Tatsuro Uchida, and Yasuhisa Adachi. 2024. "Growth of Floc Structure and Subsequence Compaction into Smaller Granules through Breakup and Rearrangement of Aluminum Flocs in a Constant Laminar Shear Flow" Gels 10, no. 1: 49. https://doi.org/10.3390/gels10010049




