Biological Activities of Ethanol Extracts of Hericium erinaceus Obtained as a Result of Optimization Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Procedure Method
2.2. Modeling
2.3. Optimization
2.4. Extraction Processes
2.5. MTT Assay
2.6. Anticholinesterase Analysis
2.7. Antimicrobial Analysis
2.8. Total Phenolic Analysis
2.9. Antioxidant Analysis
2.9.1. Total Antioxidant Status and Total Oxidant Status
2.9.2. DPPH Free Radical Scavenging Activity
2.9.3. Ferric-Reducing Antioxidant Power Assay
2.10. Phenolic Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction Conditions
3.2. Antiproliferative Activity
3.3. Anti-Alzheimer Activity
3.4. Antimicrobial Potential
3.5. Total Phenolic Contents
3.6. Antioxidant Activity
3.7. Phenolic Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-volatile taste components of several speciality mushrooms. Food Chem. 2001, 73, 461–466. [Google Scholar] [CrossRef]
- Ivanova, T.; Krupodorova, T.; Barshteyn, V.; Artamonova, A.; Shlyakhovenko, V. Anticancer substances of mushroom origin. Exp. Oncol. 2014, 36, 58–66. [Google Scholar] [PubMed]
- Lazur, J.; Hnatyk, K.; Kała, K.; Sułkowska-Ziaja, K.; Muszyńska, B. Discovering the potential mechanisms of medicinal mushrooms antidepressant activity: A review. Antioxidants 2023, 12, 623. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Song, K.; Li, L.; Chen, M. Medicinal value of edible mushroom polysaccharides: A review. J. Future Foods 2023, 3, 16–23. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Tang, L.; Xie, Y.; Xie, L. Secondary metabolites from Hericium erinaceus and their anti-inflammatory activities. Molecules 2022, 27, 2157. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E. Hericium erinaceus in neurodegenerative diseases: From bench to bedside and beyond, how far from the shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Tangen, J.-M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L. Anti-aging and neuroprotective properties of Grifola frondosa and Hericium erinaceus extracts. Nutrients 2022, 14, 4368. [Google Scholar] [CrossRef]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Antibiofilm activity and bioactive phenolic compounds of ethanol extract from the Hericium erinaceus basidiome. J. Adv. Pharm. Technol. Res. 2022, 13, 111–116. [Google Scholar] [PubMed]
- Suleiman, W.B.; Shehata, R.M.; Younis, A.M. In vitro assessment of multipotential therapeutic importance of Hericium erinaceus mushroom extracts using different solvents. Bioresour. Bioprocess. 2022, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of ultrasonic extraction to obtain erinacine a and polyphenols with antioxidant activity from the fungal biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandey, V.K.; Chakraborty, S.; Dash, K.K.; Singh, R.; Béla, K. Ultrasound-assisted extraction of phytochemicals from green coconut shell: Optimization by integrated artificial neural network and particle swarm technique. Heliyon 2023, 9, e22438. [Google Scholar] [CrossRef] [PubMed]
- Olalere, O.A.; Gan, C.Y.; Adedeji, P.A.; Olalere, M.E.; Aljbour, N. Multi-objective Deng’s grey incidence analysis, orthogonal optimization, and artificial neural network modelling in hot-maceration-assisted extraction of African cucumber leaves (Momordica balsamina). Can. J. Chem. Eng. 2022, 100, 588–597. [Google Scholar] [CrossRef]
- Pehlivan, M.; Yumrutaş, Ö.; Bozgeyik, İ. Antimutagenic and antioxidant activities of teucrium multicaule and its cytotoxic effect on murine LR7 cell. Commun. Fac. Sci. Univ. Ank. Ser. C Biol. 2020, 29, 95–104. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Hindler, J.; Hochstein, L.; Howell, A. Preparation of routine media and reagents used in antimicrobial sensitivity testing. Clin. Microbiol. Proced. Handb. 1992, 5, 1–30. [Google Scholar]
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Uysal, I. Total phenolic and flavonoid contents and antioxidant, antimicrobial and antiproliferative activities of Polycarpon tetraphyllum. Kuwait J. Sci. 2023, 50, 322–325. [Google Scholar] [CrossRef]
- Çömlekçioğlu, N.; Çırak, R. Comparison of fatty acid composition and antioxidant contents of Tribulus terrestris L. collected from different localities. Turk. J. Agric. -Food Sci. Technol. 2021, 9, 1448–1454. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.S. Phenolic Contents, Antioxidant and Antimicrobial activıties of Allium stamineum collected from duhok (Iraq). Fresenius Environ. Bull. 2020, 29, 7526–7531. [Google Scholar]
- Kalkan, M.; Aygan, A.; Çömlekçioglu, N.; Çömlekçioğlu, U. Investigation of Some bioactive properties and antimicrobial activity of Olea europaea leaves. Turk. J. Agric. —Food Sci. Technol. 2023, 11, 496–504. [Google Scholar]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Andonova, T.; Petkova, Z.; Teneva, O.; Antova, G.; Apostolova, E.; Naimov, S.; Mladenova, T.; Slavov, I.; Dimitrova-Dyulgerova, I. Ailanthus altissima seed oil—A valuable source of lipid-soluble components with dna protective and antiproliferative activities. Foods 2024, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Konrath, E.L.; Passos, C.d.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef]
- Tel, G.; Ozturk, M.; Duru, M.E.; Turkoglu, A. Antioxidant and anticholinesterase activities of five wild mushroom species with total bioactive contents. Pharm. Biol. 2015, 53, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Im, K.H.; Choi, J.; Shin, P.G.; Lee, T.S. Evaluation of antioxidant, anti-cholinesterase, and anti-inflammatory effects of culinary mushroom Pleurotus pulmonarius. Mycobiology 2016, 44, 291–301. [Google Scholar] [CrossRef]
- Garrab, M.; Edziri, H.; El Mokni, R.; Mastouri, M.; Mabrouk, H.; Douki, W. Phenolic composition, antioxidant and anticholinesterase properties of the three mushrooms Agaricus silvaticus Schaeff., Hydnum rufescens Pers. and Meripilus giganteus (Pers.) Karst. in Tunisia. S. Afr. J. Bot. 2019, 124, 359–363. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F. LC-ESI-QTOF-MS/MS analysis, cytotoxic, antiviral, antioxidant, and enzyme inhibitory properties of four extracts of Geranium pyrenaicum Burm. f.: A good gift from the natural treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Ćurlin, P.; Brzović, P.; Auzende, K.; Magné, C.; Generalić Mekinić, I. Volatiles from French and Croatian sea fennel ecotypes: Chemical profiles and the antioxidant, antimicrobial and antiageing activity of essential oils and hydrolates. Foods 2024, 13, 695. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-U.; Lee, E.-H.; Jung, H.-Y.; Lee, S.-Y.; Cho, Y.-J. Inhibitory activity against biological activities and antimicrobial activity against pathogenic bacteria of extracts from Hericium erinaceus. J. Appl. Biol. Chem. 2019, 62, 173–179. [Google Scholar] [CrossRef]
- Alkin, M.; Söğüt, E.; Seydim, A.C. Determination of bioactive properties of different edible mushrooms from Turkey. J. Food Meas. Charact. 2021, 15, 3608–3617. [Google Scholar] [CrossRef]
- Li, H.; Park, S.; Moon, B.; Yoo, Y.-B.; Lee, Y.-W.; Lee, C. Targeted phenolic analysis in Hericium erinaceum and its antioxidant activities. Food Sci. Biotechnol. 2012, 21, 881–888. [Google Scholar] [CrossRef]
- Kopylchuk, H.; Voloshchuk, O.; Pasailiuk, M. Comparison of total amino acid compositions, total phenolic compounds, total flavonoid content, β-carotene content and hydroxyl radical scavenging activity in four wild edible mushrooms. Ital. J. Mycol. 2023, 52, 112–125. [Google Scholar]
- Shomali, N.; Onar, O.; Karaca, B.; Demirtas, N.; Cihan, A.C.; Akata, I.; Yildirim, O. Antioxidant, anticancer, antimicrobial, and antibiofilm properties of the culinary-medicinal fairy ring mushroom, Marasmius oreades (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 571–582. [Google Scholar] [CrossRef]
- Yu, M.; Wang, B.; Huang, Z.; Lv, J.; Teng, Y.; Li, T.; Zhang, Y.; Dong, K.; Qin, D.; Huo, J. Evaluation of blue honeysuckle berries (Lonicera caerulea L.) dried at different temperatures: Basic quality, sensory attributes, bioactive compounds, and in vitro antioxidant activity. Foods 2024, 13, 1240. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.I.; Bal, C.; Eraslan, E.C.; Sevindik, M.; Akgul, H. Biological activities of Agrocybe praecox (spring fieldcap mushroom). Prospect. Pharm. Sci. 2023, 21, 33–39. [Google Scholar] [CrossRef]
- Karaltı, İ.; Eraslan, E.C.; Sarıdoğan, B.G.Ö.; Akata, I.; Sevindik, M. Total antioxidant, antimicrobial, antiproliferative potentials and element contents of wild mushroom Candolleomyces candolleanus (Agaricomycetes) from Turkey. Int. J. Med. Mushrooms 2022, 24, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bal, C.; Baba, H.; Akata, I.; Sevindik, M.; Selamoglu, Z.; Akgül, H. Biological activities of wild poisonous mushroom Entoloma sinuatum (Bull.) P. Kumm (Boletales). KSU J. Agric. Nat. 2022, 25, 83–87. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. The addition of reishi and lion’s mane mushroom powder to pasta influences the content of bioactive compounds and the antioxidant, potential anti-inflammatory, and anticancer properties of pasta. Antioxidants 2023, 12, 738. [Google Scholar] [CrossRef]

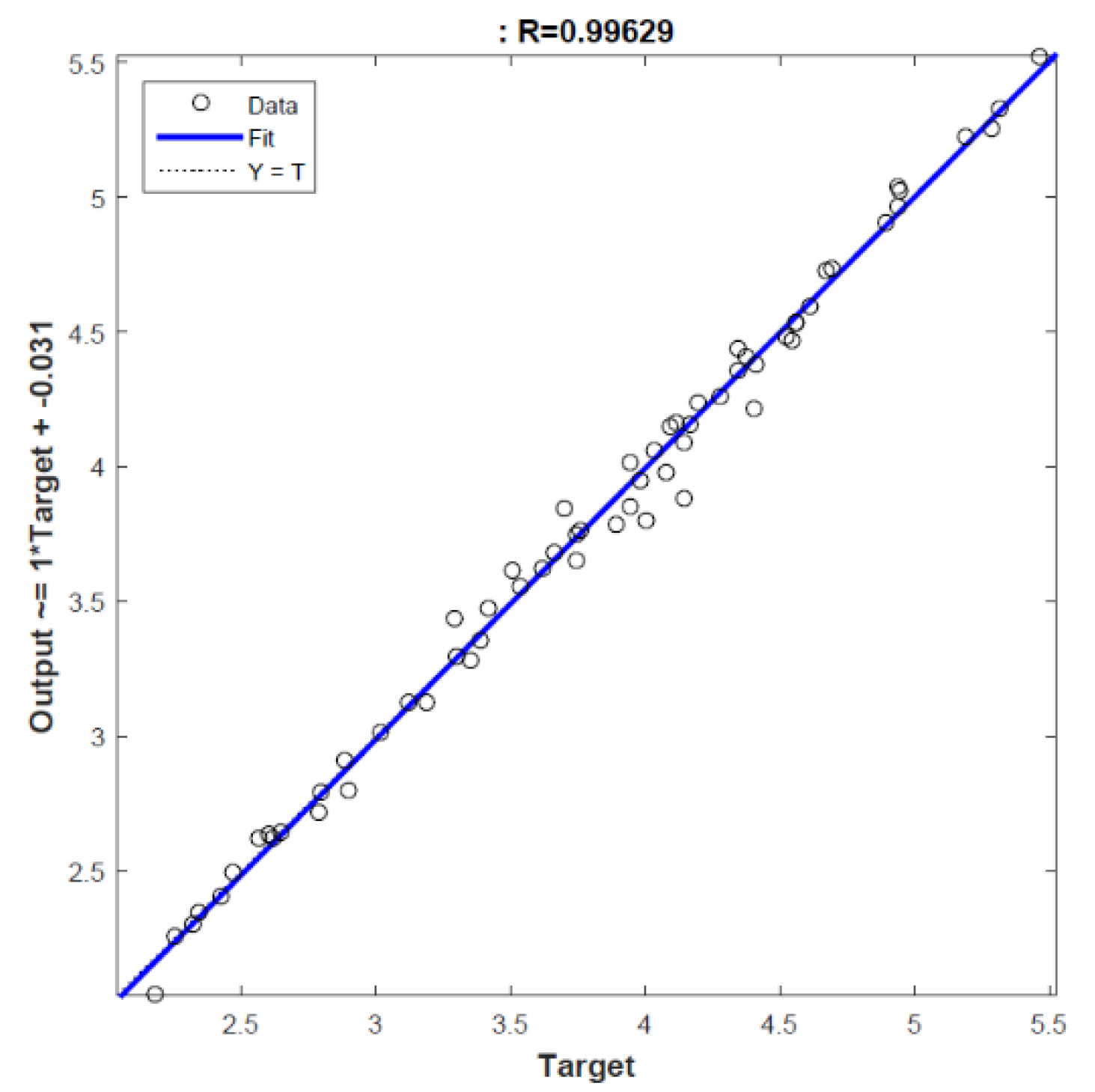
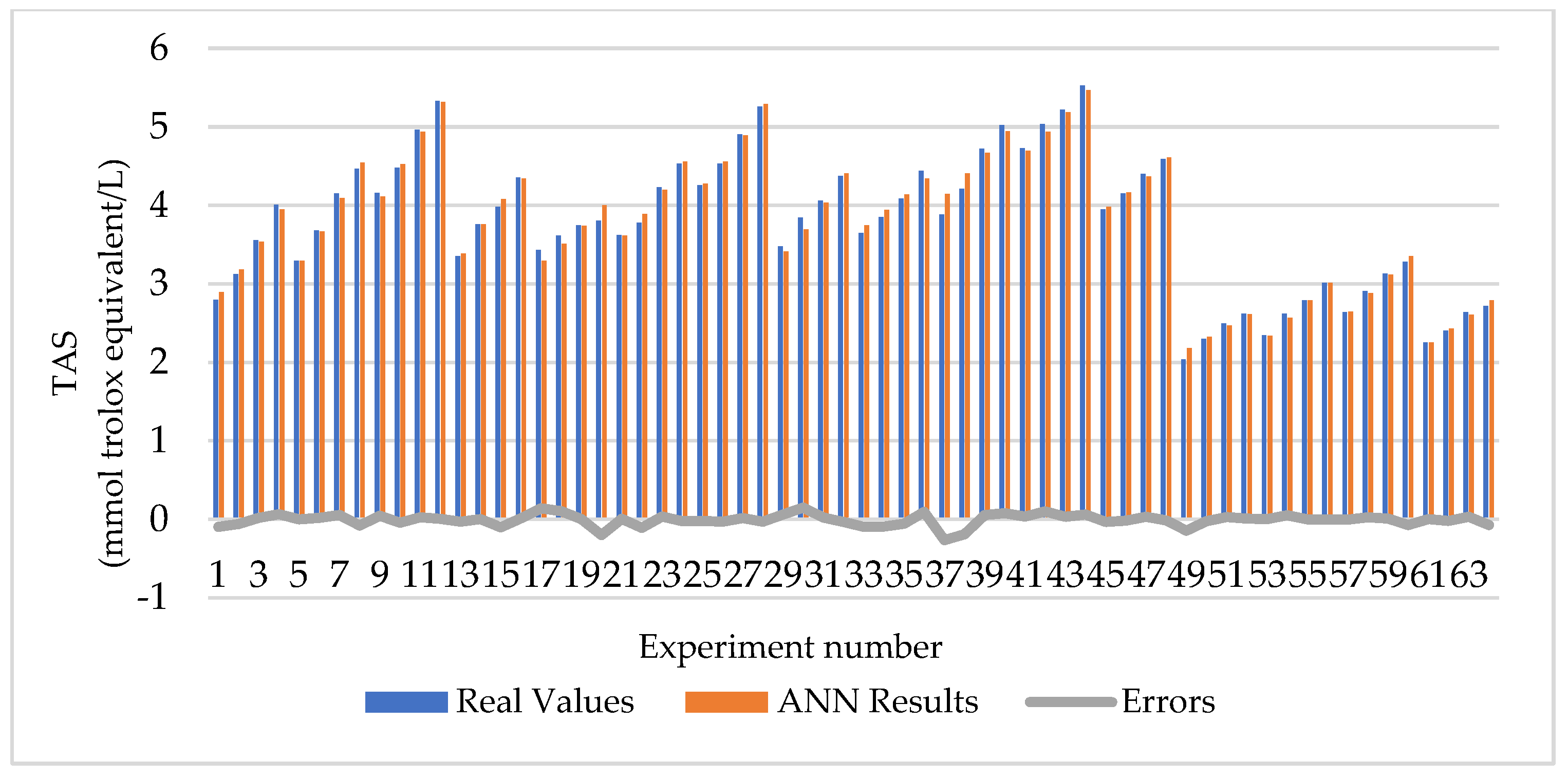


| Experiment Number | Extraction Temperature (°C) | Extraction Time (h) | Extract Concentration (mg/mL) | TAS (mmol Trolox Equivalent/L) |
|---|---|---|---|---|
| 1 | 40 | 3 | 0.25 | 2.799 ± 0.233 |
| 2 | 0.5 | 3.127 ± 0.121 | ||
| 3 | 1 | 3.556 ± 0.074 | ||
| 4 | 2 | 4.011 ± 0.173 | ||
| 5 | 5 | 0.25 | 3.297 ± 0.106 | |
| 6 | 0.5 | 3.683 ± 0.115 | ||
| 7 | 1 | 4.149 ± 0.119 | ||
| 8 | 2 | 4.465 ± 0.212 | ||
| 9 | 7 | 0.25 | 4.159 ± 0.186 | |
| 10 | 0.5 | 4.480 ± 0.141 | ||
| 11 | 1 | 4.965 ± 0.076 | ||
| 12 | 2 | 5.325 ± 0.125 | ||
| 13 | 9 | 0.25 | 3.355 ± 0.139 | |
| 14 | 0.5 | 3.759 ± 0.115 | ||
| 15 | 1 | 3.979 ± 0.086 | ||
| 16 | 2 | 4.356 ± 0.205 | ||
| 17 | 50 | 3 | 0.25 | 3.433 ± 0.088 |
| 18 | 0.5 | 3.612 ± 0.059 | ||
| 19 | 1 | 3.746 ± 0.066 | ||
| 20 | 2 | 3.803 ± 0.080 | ||
| 21 | 5 | 0.25 | 3.624 ± 0.033 | |
| 22 | 0.5 | 3.782 ± 0.073 | ||
| 23 | 1 | 4.234 ± 0.066 | ||
| 24 | 2 | 4.535 ± 0.070 | ||
| 25 | 7 | 0.25 | 4.260 ± 0.072 | |
| 26 | 0.5 | 4.530 ± 0.119 | ||
| 27 | 1 | 4.907 ± 0.064 | ||
| 28 | 2 | 5.254 ± 0.122 | ||
| 29 | 9 | 0.25 | 3.475 ± 0.049 | |
| 30 | 0.5 | 3.846 ± 0.103 | ||
| 31 | 1 | 4.058 ± 0.084 | ||
| 32 | 2 | 4.374 ± 0.063 | ||
| 33 | 60 | 3 | 0.25 | 3.651 ± 0.118 |
| 34 | 0.5 | 3.851 ± 0.089 | ||
| 35 | 1 | 4.086 ± 0.142 | ||
| 36 | 2 | 4.439 ± 0.105 | ||
| 37 | 5 | 0.25 | 3.882 ± 0.098 | |
| 38 | 0.5 | 4.214 ± 0.168 | ||
| 39 | 1 | 4.725 ± 0.158 | ||
| 40 | 2 | 5.020 ± 0.113 | ||
| 41 | 7 | 0.25 | 4.731 ± 0.091 | |
| 42 | 0.5 | 5.035 ± 0.061 | ||
| 43 | 1 | 5.221 ± 0.093 | ||
| 44 | 2 | 5.323 ± 0.109 | ||
| 45 | 9 | 0.25 | 3.950 ± 0.102 | |
| 46 | 0.5 | 4.152 ± 0.094 | ||
| 47 | 1 | 4.404 ± 0.046 | ||
| 48 | 2 | 4.594 ± 0.090 | ||
| 49 | 70 | 3 | 0.25 | 2.038 ± 0.096 |
| 50 | 0.5 | 2.301 ± 0.107 | ||
| 51 | 1 | 2.496 ± 0.059 | ||
| 52 | 2 | 2.623 ± 0.118 | ||
| 53 | 5 | 0.25 | 2.344 ± 0.127 | |
| 54 | 0.5 | 2.619 ± 0.089 | ||
| 55 | 1 | 2.791 ± 0.059 | ||
| 56 | 2 | 3.010 ± 0.119 | ||
| 57 | 7 | 0.25 | 2.643 ± 0.063 | |
| 58 | 0.5 | 2.906 ± 0.095 | ||
| 59 | 1 | 3.128 ± 0.051 | ||
| 60 | 2 | 3.282 ± 0.074 | ||
| 61 | 9 | 0.25 | 2.256 ± 0.059 | |
| 62 | 0.5 | 2.407 ± 0.064 | ||
| 63 | 1 | 2.638 ± 0.092 | ||
| 64 | 2 | 2.717 ± 0.110 |
| AChE (μg/mL) | BChE (μg/mL) | |
|---|---|---|
| Ethanol | 13.85 ± 0.94 b* | 28.00 ± 0.89 b |
| Galantamine | 6.21 ± 0.62 a | 25.01 ± 0.43 a |
| S. aureus | S. aureus MRSA | E. faecalis | E. coli | P. aeruginosa | A. baumannii | C. glabrata | C. albicans | C. krusei | |
|---|---|---|---|---|---|---|---|---|---|
| Ethanol extract | 25 | 25 | 50 | 100 | 100 | 100 | 100 | 50 | 100 |
| Ampicillin | 1.56 | 3.12 | 1.56 | 3.12 | 3.12 | - | - | - | - |
| Amikacin | - | - | - | 1.56 | 3.12 | 3.12 | - | - | - |
| Ciprofloksasin | 1.56 | 3.12 | 1.56 | 1.56 | 3.12 | 3.12 | - | - | - |
| Flukanazol | - | - | - | - | - | - | 3.12 | 3.12 | - |
| Amfoterisin B | - | - | - | - | - | - | 3.12 | 3.12 | 3.12 |
| TAS (mmol/L) | 5.426 ± 0.123 |
| TOS (µmol/L) | 6.621 ± 0.197 |
| OSI (TOS/(TAS × 10)) | 0.122 ± 0.003 |
| TPC (mg/g) | 59.75 ± 1.82 |
| DPPH (mg Trolox equivalent/g) | 73.36 ± 2.04 |
| FRAP (mg Trolox equivalent/g) | 107.66 ± 2.41 |
| Phenolic Compounds | Values (mg/kg) |
|---|---|
| Acetohydroxamic acid | 2.46415 |
| Catechinhyrate | 17.54653 |
| Vanillic acid | <LD |
| Syringic acid | <LD |
| Thymoquinone | <LD |
| Resveratrol | 9.4593 |
| Myricetin | 1.361 |
| Kaempferol | <LD |
| Fumaric acid | 9.39341 |
| Gallic acid | 92.00393 |
| Protocatechuic acid | 3.8488 |
| 4-hydroxybenzoic acid | 0.40874 |
| Caffeic acid | <LD |
| Salicylic acid | <LD |
| Phloridzindyhrate | 0.23083 |
| 2-hydoxycinamic acid | 0.94018 |
| Oleuropein | <LD |
| 2-hyroxy1,4 naphthaquinone | <LD |
| Naringenin | 0.42601 |
| Silymarin | <LD |
| Quercetin | 2.34281 |
| Luteolin | 0.44483 |
| Alizarin | <LD |
| Curmin | <LD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevindik, M.; Gürgen, A.; Khassanov, V.T.; Bal, C. Biological Activities of Ethanol Extracts of Hericium erinaceus Obtained as a Result of Optimization Analysis. Foods 2024, 13, 1560. https://doi.org/10.3390/foods13101560
Sevindik M, Gürgen A, Khassanov VT, Bal C. Biological Activities of Ethanol Extracts of Hericium erinaceus Obtained as a Result of Optimization Analysis. Foods. 2024; 13(10):1560. https://doi.org/10.3390/foods13101560
Chicago/Turabian StyleSevindik, Mustafa, Ayşenur Gürgen, Vadim Tagirovich Khassanov, and Celal Bal. 2024. "Biological Activities of Ethanol Extracts of Hericium erinaceus Obtained as a Result of Optimization Analysis" Foods 13, no. 10: 1560. https://doi.org/10.3390/foods13101560





