Formation and Application of Starch–Polyphenol Complexes: Influencing Factors and Rapid Screening Based on Chemometrics
Abstract
:1. Introduction
2. Effects of Polyphenols on the Formation of Starch–Polyphenol Complexes
2.1. Catalogue of Polyphenols
2.2. Molecular Weight of Polyphenols
2.3. Phenolic Hydroxyl Groups
2.3.1. Number of Hydroxyl Groups
2.3.2. Spatial Distribution of Phenolic Groups
2.4. Methoxy Group
2.5. Concentration of Polyphenols
2.6. Polyphenols from Different Botanical Sources
2.6.1. Tea Polyphenols
2.6.2. Cereal Polyphenols
3. Effect of Starch Properties on the Formation of Starch–Polyphenol Complexes
3.1. Amylose/Amylopectin Ratio
3.2. Different Starch Crystalline Structures from Different Botanical Backgrounds
3.2.1. A-Type Cereal Starch
3.2.2. B-Type Tubers Starch
3.2.3. C-Type Starch
3.3. Various Botanical Sources of Starch
3.3.1. Maize Starch
3.3.2. Rice Starch
4. Effect of Processing on the Formation of Starch–Polyphenol Complexes
4.1. The pH Condition of Starch and Polyphenols
4.2. The Processing Methods of Starch–Polyphenol Complexes
4.2.1. Microwave Processing
4.2.2. Ultrasound Processing
4.2.3. Extrusion Processing
5. Polyphenol and Starch Diversity: Rapid Identification Based on Spectroscopy Techniques Coupled with Chemometrics
5.1. Rapid Identification of Amylose Content in Starch
5.1.1. NIR Spectra
5.1.2. Raman Spectra
5.2. Phenolic Content
6. Application of Starch–Polyphenol Complexes in the Food Industry
6.1. Starch as an Encapsulation Agent
6.2. Starch–Polyphenol Complexes and Their Influence on Digestibility
6.2.1. Polyphenol-Mediated Inhibition of Digestive Enzymes
6.2.2. The Role of Starch Structure in the Starch–Polyphenol Complex
6.3. Modifier of Food Quality
6.4. Application of Starch–Polyphenol Complexes in Starch Products
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; He, Z.; Yang, L.; Wang, H.; Lian, X.; Zhu, W. Retrogradation of sweet potato amylose and amylopectin with narrow molecular weight distribution. Int. J. Food Sci. Technol. 2022, 57, 1954–1964. [Google Scholar] [CrossRef]
- Kasaai, M.R. A comparative study of molecular structures, solution properties and food applications for three branched polysaccharides: Amylopectin, glycogen, and dextran. Polymer 2012, 16, 49–63. [Google Scholar]
- Tan, L.; Kong, L. Starch-guest inclusion complexes: Formation, structure, and enzymatic digestion. Crit. Rev. Food Sci. Nutr. 2020, 60, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.; Chen, C.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Martínez, M.M. Unraveling the Inhibition of Intestinal Glucose Transport by Dietary Phenolics: A Review. Curr. Pharm. Design 2019, 25, 3418–3433. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Capuano, E.; Oliviero, T.; Renzetti, S. Wheat starch-tannic acid complexes modulate physicochemical and rheological properties of wheat starch and its digestibility. Food Hydrocoll. 2022, 126, 107459. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Li, L.; Bodjrenou, D.M.; Lu, X.; Zheng, B. Effect of chlorogenic acid on lotus seed starch gelatinization behavior and complexation mode during microwave treatment. Food Hydrocoll. 2023, 144, 108925. [Google Scholar] [CrossRef]
- Li, M.; Ndiaye, C.; Corbin, S.; Foegeding, E.A.; Ferruzzi, M.G. Starch-phenolic complexes are built on physical CH-π interactions and can persist after hydrothermal treatments altering hydrodynamic radius and digestibility of model starch-based foods. Food Chem. 2020, 308, 125577. [Google Scholar] [CrossRef]
- Amoako, D.B.; Awika, J.M. Resistant starch formation through intrahelical V-complexes between polymeric proanthocyanidins and amylose. Food Chem. 2019, 285, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, B.; Chen, J.; Chen, L.; Zheng, B. Insight into the characterization and digestion of lotus seed starch-tea polyphenol complexes prepared under high hydrostatic pressure. Food Chem. 2019, 297, 124992. [Google Scholar] [CrossRef]
- Amoako, D.; Awika, J.M. Polyphenol interaction with food carbohydrates and consequences on availability of dietary glucose. Curr. Opin. Food Sci. 2016, 8, 14–18. [Google Scholar] [CrossRef]
- Amoako, D.B.; Awika, J.M. Polymeric tannins significantly alter properties and in vitro digestibility of partially gelatinized intact starch granule. Food Chem. 2016, 208, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, M.; Zhang, R.; Huang, L.; Jia, X.; Huang, F.; Liu, L. Physicochemical interactions between rice starch and different polyphenols and structural characterization of their complexes. LWT-Food Sci. Technol. 2020, 125, 109227. [Google Scholar] [CrossRef]
- Han, M.; Bao, W.; Wu, Y.; Ouyang, J. Insights into the effects of caffeic acid and amylose on in vitro digestibility of maize starch-caffeic acid complex. Int. J. Biol. Macromol. 2020, 162, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.F.; De Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Li, Z.; Gao, Y.; Cui, S.W.; Wang, T.; Qiu, J. Diverse effects of rutin and quercetin on the pasting, rheological and structural properties of Tartary buckwheat starch. Food Chem. 2021, 335, 127556. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Feng, Z.-J.; Gao, H.-X.; He, Q.; Zeng, W.-C. Elucidating the influence and mechanism of different phenols on the properties, food quality and function of maize starch. Food Chem. 2024, 449, 139191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, B.; Du, A.; Chen, J.; Chen, L. Insight into the retardation of retrogradation of chestnut starch by heat-moisture treatment with flavonoids. Food Chem. 2023, 404, 134587. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.S.; Guo, J.Y.; Feng, J.N.; Tan, L.B.; Kong, L.Y. Inhibition of starch digestion by gallic acid and alkyl gallates. Food Hydrocoll. 2020, 102, 105603. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.-X.; He, Q.; Yu, Z.-L.; Zeng, W.-C. Influence of structure complexity of phenolic compounds on their binding with maize starch. Food Struct. 2022, 33, 100286. [Google Scholar] [CrossRef]
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose Structural Characteristics, Methods of Preparation, Significance, and Potential Applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Fan, H.; Chen, Z.; Ma, R.; Wen, Y.; Li, H.; Wang, J.; Sun, B. V6a-amylose helical cavity and benzoic acids with para-hydroxyl structure facilitate the formation of inclusion complex. Carbohydr. Polym. 2022, 298, 120065. [Google Scholar] [CrossRef]
- Fan, H.; Chen, Z.; Xu, L.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Both alkyl chain length and V-amylose structure affect the structural and digestive stability of amylose-alkylresorcinols inclusion complexes. Carbohydr. Polym. 2022, 292, 119567. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Q.; Li, R.; Chen, L.; Zheng, B. Amylose content controls the V-type structural formation and in vitro digestibility of maize starch-resveratrol complexes and their effect on human gut microbiota. Carbohydr. Polym. 2024, 327, 121702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, X.; Guo, Z.; Zheng, B.; Zhang, Y. Insights into the multi-scale structural properties and digestibility of lotus seed starch-chlorogenic acid complexes prepared by microwave irradiation. Food Chem. 2021, 361, 130171. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Xiong, L.; Li, M.; Wang, Y.; Lin, M.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Interactions between debranched starch and emulsifiers, polyphenols, and fatty acids. Int. J. Biol. Macromol. 2020, 150, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, M.; Pan, J.; Zhang, S.; Jiang, Y.; Liu, J.; Zhu, Y.; Zhang, H. Interactions between tea products and wheat starch during retrogradation. Food Biosci. 2020, 34, 100523. [Google Scholar] [CrossRef]
- Liu, B.; Zhong, F.; Yokoyama, W.; Huang, D.; Zhu, S.; Li, Y. Interactions in starch co-gelatinized with phenolic compound systems: Effect of complexity of phenolic compounds and amylose content of starch. Carbohydr. Polym. 2020, 247, 116667. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, Z.; Xu, X.; Wang, X.; Du, X. Effects of tea polyphenols on the structural and physicochemical properties of high-hydrostatic-pressure-gelatinized rice starch. Food Hydrocoll. 2019, 91, 256–262. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Ogawa, Y.; Kong, X.; Chen, S.; Liu, D.; Ye, X. Physicochemical properties and in vitro digestion of extruded rice with grape seed proanthocyanidins. J. Cereal Sci. 2020, 95, 103064. [Google Scholar] [CrossRef]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Feng, Z.-J.; Gao, H.-X.; He, Q.; Zeng, W.-C. Effects of phenols with different structure characteristics on properties of potato starch: Action rule and molecular mechanism. J. Food Process Preserv. 2022, 46, e16679. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G. A Review of Dietary Polyphenol-Plasma Protein Interactions: Characterization, Influence on the Bioactivity, and Structure-Affinity Relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhu, S.; Li, Y.; Zhong, F.; Huang, D.; Chen, X. Role of phenolic acids with different functional groups in the regulation of starch digestion in simulated dietary intake patterns. Int. J. Biol. Macromol. 2023, 235, 123815. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Ren, Y.; Ye, X.; Kong, X.; Tian, J. Regulating the physicochemical, structural characteristics and digestibility of potato starch by complexing with different phenolic acids. Int. J. Biol. Macromol. 2023, 253, 127474. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhu, S.; Zhong, F.; Zhang, S.; Du, C.; Li, Y. Insight into the multi-scale structure changes and mechanism of corn starch modulated by different structural phenolic acids during retrogradation. Food Hydrocoll. 2022, 128, 107581. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.-X.; He, Q.; Zeng, W.-C. Insight into property, function, and digestion of potato starch modified by phenolic compounds with varying structures. J. Food Sci. 2023, 88, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yao, X.; Chen, Z.; Ma, R.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Interaction of high amylose corn starch with polyphenols: Modulating the stability of polyphenols with different structure against thermal processing. Food Chem. 2024, 437, 137708. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zheng, M.; Yang, S.; Li, Z.; Liu, M.; Yang, X.; Lin, N.; Liu, J. Physicochemical properties and in vitro digestibility of proso millet starch after addition of Proanthocyanidins. Int. J. Biol. Macromol. 2021, 168, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, P.; Chen, X.D. Mechanistic insights into the influence of flavonoids from dandelion on physicochemical properties and in vitro digestibility of cooked potato starch. Food Hydrocoll. 2022, 130, 107714. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Liu, G.-Q.; Wu, Y.; Tian, W.; Wu, W.; Fu, X. Effect of green tea polyphenols on the gelatinization and retrogradation of rice starches with different amylose contents. J. Med. Plants Res. 2011, 5, 4298–4303. [Google Scholar]
- Liu, W.; Xu, J.; Shuai, X.; Geng, Q.; Guo, X.; Chen, J.; Li, T.; Liu, C.; Dai, T. The interaction and physicochemical properties of the starch-polyphenol complex: Polymeric proanthocyanidins and maize starch with different amylose/amylopectin ratios. Int. J. Biol. Macromol. 2023, 253, 126617. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chen, L.; Gao, H.X.; Zeng, W.C. Mechanism of bridging and interfering effects of tea polyphenols on starch molecules. J. Food Process Preserv. 2020, 44, e14576. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, Y.-J. Rheological and thermal properties of rice starch and rutin mixtures. Food Res. Int. 2012, 49, 757–762. [Google Scholar] [CrossRef]
- Wu, X.; Fu, G.; Li, R.; Li, Y.; Dong, B.; Liu, C. Effect of thermal processing for rutin preservation on the properties of phenolics & starch in Tartary buckwheat achenes. Int. J. Biol. Macromol. 2020, 164, 1275–1283. [Google Scholar] [PubMed]
- Li, H.; Zhai, F.; Li, J.; Zhu, X.; Guo, Y.; Zhao, B.; Xu, B. Physicochemical properties and structure of modified potato starch granules and their complex with tea polyphenols. Int. J. Biol. Macromol. 2021, 166, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, L.; Li, M.; He, X.; Hao, L.; Dai, Y. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols. Int. J. Biol. Macromol. 2019, 129, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Guzar, I.; Ragaee, S.; Seetharaman, K. Mechanism of Hydrolysis of Native and Cooked Starches from Different Botanical Sources in the Presence of Tea Extracts. J. Food Sci. 2012, 77, C1192–C1196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lin, Q.; Liu, G.-Q.; Wu, Y.; Wu, W.; Fu, X. Inhibitory Effects of Green Tea Polyphenols on the Retrogradation of Starches from Different Botanical Sources. Food Bioprocess Technol. 2013, 6, 2177–2181. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, C.; Zhang, H.; Jiang, H. Characteristics of three typical Chinese highland barley varieties: Phenolic compounds and antioxidant activities. J. Food Biochem. 2018, 42, e12488. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Rong, L.; Chen, Y.; Yu, Q.; Shen, M.; Xie, J. Purple red rice bran anthocyanins reduce the digestibility of rice starch by forming V-type inclusion complexes. Food Res. Int. 2023, 166, 112578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Van Haute, M.; Korth, N.; Sattler, S.; Rose, D.; Juritsch, A.; Shao, J.; Beede, K.; Schmaltz, R.; Price, J.; et al. The waxy mutation in sorghum and other cereal grains reshapes the gut microbiome by reducing levels of multiple beneficial species. Gut Microbes 2023, 15, 2178799. [Google Scholar] [CrossRef] [PubMed]
- Tejavathi, D.; Sujatha, B.; Karigar, C. Physicochemical properties of starch obtained from Curcuma karnatakensis-A new botanical source for high amylose content. Heliyon 2020, 6, e03169. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Z.; Chen, R.; Wang, Z.; Zhang, S.; Chen, J. Probing molecular interactions of amylose-morin complex and their effect on antioxidant capacity by 2D solid-state NMR spectroscopy. Food Chem. 2023, 415, 135693. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between Amylose and Tea Polyphenols Modulates the Postprandial Glycemic Response to High-Amylose Maize Starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Z.; Wang, Z.; Chen, R.; Zhang, S. Molecular interactions between apigenin and starch with different amylose/amylopectin ratios revealed by X-ray diffraction, FT-IR and solid-state NMR. Carbohydr. Polym. 2023, 310, 120737. [Google Scholar] [CrossRef]
- Liu, R.; Xu, C.; Cong, X.; Wu, T.; Song, Y.; Zhang, M. Effects of oligomeric procyanidins on the retrogradation properties of maize starch with different amylose/amylopectin ratios. Food Chem. 2017, 221, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, P. Botanical sources of starch. In Starch Structure, Functionality and Application in Foods; Springer: Berlin/Heidelberg, Germany, 2020; pp. 9–27. [Google Scholar]
- Li, Q.; Guo, A.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Tunable interactions in starch-anthocyanin complexes switched by high hydrostatic pressure. Food Chem. 2024, 436, 137677. [Google Scholar] [CrossRef]
- Sengupta, A.; Chakraborty, I.; Mazumder, N. An insight into the physicochemical characterisation of starch-lipid complex and its importance in food industry. Food Rev. Int. 2023, 39, 4198–4212. [Google Scholar] [CrossRef]
- Zhang, X.; Rehman, R.-u.; Wang, S.; Ji, Y.; Li, J.; Liu, S.; Wang, H. Blue honeysuckle extracts retarded starch digestion by inhibiting glycosidases and changing the starch structure. Food Funct. 2022, 13, 6072–6088. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Kong, X.; Wu, D.; Chen, S.; Liu, D.; Ye, X. Proanthocyanidins from Chinese berry leaves modified the physicochemical properties and digestive characteristic of rice starch. Food Chem. 2021, 335, 127666. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, H.; Sun, L.; Cao, J.; Yang, J.; Lu, M.; Wang, M. Rheological, thermal and in vitro digestibility properties on complex of plasma modified Tartary buckwheat starches with quercetin. Food Hydrocoll. 2021, 110, 106209. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Xu, H.; Liang, Y.; Zheng, B. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization. Int. J. Biol. Macromol. 2019, 134, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, S.; Xie, H.; Zhang, Y.; Fu, L. Polyphenols from pigmented quinoa as potential modulators of maize starch digestion: Role of the starch-polyphenol inclusion and non-inclusion complexes. Food Hydrocoll. 2023, 144, 108975. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, T.; Li, Z.; Gao, Y.; Cui, S.W.; Qiu, J. Comparison of quercetin and rutin inhibitory influence on Tartary buckwheat starch digestion in vitro and their differences in binding sites with the digestive enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, C.; Liu, Z.; Xu, H.; Wei, B.; Wang, B.; Sun, Q.; Zhou, C.; Ma, H. Starches modification with rose polyphenols under multi-frequency power ultrasonic fields: Effect on physicochemical properties and digestion behavior. Ultrason. Sonochem. 2023, 98, 106515. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dai, T.; Chen, J.; He, X.; Shuai, X.; Liu, C.; Li, T. Effects of Three Types of Polymeric Proanthocyanidins on Physicochemical and In Vitro Digestive Properties of Potato Starch. Foods 2021, 10, 1394. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Fang, H.; Zhang, H.; Kong, X.; Wu, D.; Zheng, J.; Liu, D.; Ye, X.; Chen, S. Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins. Molecules 2020, 25, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, B.; Zheng, B.; Chen, L.; Guo, Z. Effects and mechanism of high-pressure homogenization on the characterization and digestion behavior of lotus seed starch–green tea polyphenol complexes. J. Funct. Food. 2019, 57, 173–181. [Google Scholar] [CrossRef]
- Cui, X.-R.; Wang, Y.-S.; Chen, Y.; Mu, H.-Y.; Chen, H.-H. Understanding the digestibility of wheat starch- caffeic acid complexes prepared by hot-extrusion 3D printing technology. Food Hydrocoll. 2023, 141, 108692. [Google Scholar] [CrossRef]
- Li, Y.; Niu, L.; Sun, C.; Tu, J.; Xiao, J. Comparison of in vitro starch digestibility and structure of matcha-fortified starch vermicelli from different botanical sources. J. Sci. Food Agric. 2023, 103, 7775–7784. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, T.; Deng, S.; Liu, H.; Yu, W. Variations in the effects of extrusion treatments and ferulic acid addition on starch digestibility with different botanical backgrounds. Carbohydr. Polym. 2024, 329, 121768. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Ou, Y.; Zheng, B. Effect of chlorogenic acid on the structural properties and digestibility of lotus seed starch during microwave gelatinization. Int. J. Biol. Macromol. 2021, 191, 474–482. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Guo, Z.; Zheng, B.; Zhang, Y. Long-term retrogradation behavior of lotus seed starch-chlorogenic acid mixtures after microwave treatment. Food Hydrocoll. 2021, 121, 106994. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT-Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]
- Igoumenidis, P.E.; Zoumpoulakis, P.; Karathanos, V.T. Physicochemical interactions between rice starch and caffeic acid during boiling. Food Res. Int. 2018, 109, 589–595. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Li, X.; Li, M. Effect of tea polyphenols on the retrogradation of rice starch. Food Res. Int. 2009, 42, 221–225. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Xie, F.; Li, L.; Bai, G. Modulating the in vitro digestibility and predicted glycemic index of rice starch gels by complexation with gallic acid. Food Hydrocoll. 2019, 89, 821–828. [Google Scholar] [CrossRef]
- Tong, Y.; Deng, H.; Kong, Y.; Tan, C.; Chen, J.; Wan, M.; Wang, M.; Yan, T.; Meng, X.; Li, L. Stability and structural characteristics of amylopectin nanoparticle-binding anthocyanins in Aronia melanocarpa. Food Chem. 2020, 311, 125687. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Insights into pH-modulated interactions between native potato starch and cyanidin-3-O-glucoside: Electrostatic interaction-dependent binding. Food Res. Int. 2022, 156, 111129. [Google Scholar] [CrossRef]
- Li, Y.; Yu, T.; Wang, Z.; Li, Q.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. The influence mechanism of pH and hydrothermal processing on the interaction between cyanidin-3-O-glucoside and starch. Food Hydrocoll. 2023, 136, 108234. [Google Scholar] [CrossRef]
- Li, M.; Griffin, L.E.; Corbin, S.; Neilson, A.P.; Ferruzzi, M.G. Modulating Phenolic Bioaccessibility and Glycemic Response of Starch-Based Foods in Wistar Rats by Physical Complexation between Starch and Phenolic Acid. J. Agric. Food Chem. 2020, 68, 13257–13266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, J.; Wang, S.; Zhou, Y. Effect of high hydrostatic pressure treatment on the formation and in vitro digestion of Tartary buckwheat starch/flavonoid complexes. Food Chem. 2022, 382, 132324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Li, L.; Zheng, B.; Zheng, S.; Lu, X. Microwave-Induced Behavior and Digestive Properties of the Lotus Seed Starch—Chlorogenic Acid Complex. Foods 2023, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, X.; Zheng, B.; Zhang, Y. Structural and physicochemical properties of lotus seed starch-chlorogenic acid complexes prepared by microwave irradiation. J. Food Sci. Technol. 2021, 58, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, L.; Fang, Y.; Chen, W.; Hunag, G. Ultrasonic-Assisted Preparation of Maize Starch–Caffeic Acid Complex: Physicochemical and Digestion Properties. Starch-Stärke 2021, 73, 2000084. [Google Scholar] [CrossRef]
- Raza, H.; Li, S.; Zhou, Q.; He, J.; Cheng, K.W.; Dai, S.; Wang, M. Effects of ultrasound-induced V-type rice starch-tannic acid interactions on starch in vitro digestion and multiscale structural properties. Int. J. Biol. Macromol. 2023, 246, 125619. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Pan, Y.-g.; Shang, W.-T.; Zhong, G.; Huang, W.-Y.; Xiang, D.; Pan, F.; Zhang, W.-m. Ultrasonic-assisted binding of canistel (Lucuma nervosa A.DC) seed starch with quercetin. Ultrason. Sonochem. 2023, 96, 106417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, X.; Kong, X.; Chen, S.; Xu, E.; Liu, D.; Ogawa, Y.; Ye, X.; Tian, J. Introduction of chlorogenic acid during extrusion affects the physicochemical properties and enzymatic hydrolysis of rice flour. Food Hydrocoll. 2021, 116, 106652. [Google Scholar] [CrossRef]
- Li, J.; Danao, M.-G.C.; Chen, S.-F.; Li, S.; Singh, V.; Brown, P.J. Prediction of Starch Content and Ethanol Yields of Sorghum Grain Using near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2015, 23, 85–92. [Google Scholar] [CrossRef]
- Xie, L.H.; Tang, S.Q.; Chen, N.; Luo, J.; Jiao, G.A.; Shao, G.N.; Wei, X.J.; Hu, P.S. Optimisation of near-infrared reflectance model in measuring protein and amylose content of rice flour. Food Chem. 2014, 142, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; He, X.; Duan, B.; Tang, S.; Luo, J.; Jiao, G.; Shao, G.; Wei, X.; Sheng, Z.; Hu, P. Optimization of Near-Infrared Reflectance Model in Measuring Gelatinization Characteristics of Rice Flour with a Rapid Viscosity Analyzer (RVA) and Differential Scanning Calorimeter (DSC). Cereal Chem. 2015, 92, 522–528. [Google Scholar] [CrossRef]
- Visnupriyan, R.; Flanagan, B.M.; Harper, K.J.; Cozzolino, D. Near infrared spectroscopy combined with chemometrics as tool to monitor starch hydrolysis. Carbohydr. Polym. 2024, 324, 121469. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, T.B.; Sharma, S.; Chattopadhyay, K. Development of NIRS models to predict protein and amylose content of brown rice and proximate compositions of rice bran. Food Chem. 2016, 191, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-H.; Tang, S.-Q.; Wei, X.-J.; Sheng, Z.-H.; Shao, G.-N.; Jiao, G.-A.; Hu, S.-K.; Wang, L.; Hu, P.-S. Simultaneous determination of apparent amylose, amylose and amylopectin content and classification of waxy rice using near-infrared spectroscopy (NIRS). Food Chem. 2022, 388, 132944. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, P.S.; Soares, A.; Castanho, A.; Almeida, A.S.; Oliveira, J.; Brites, C. Optimization of rice amylose determination by NIR-spectroscopy using PLS chemometrics algorithms. Food Chem. 2018, 242, 196–204. [Google Scholar] [CrossRef]
- Ichinose, J.; Oba, K.; Arase, Y.; Kaneshiro, J.; Tate, S.-i.; Watanabe, T.M. Quantitative prediction of rice starch digestibility using Raman spectroscopy and multivariate calibration analysis. Food Chem. 2024, 435, 137505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. TrAC Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.L.R.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Rizvi, T.S.; Mabood, F.; Ali, L.; Al-Broumi, M.; Al Rabani, H.K.M.; Hussain, J.; Jabeen, F.; Manzoor, S.; Al-Harrasi, A. Application of NIR Spectroscopy Coupled with PLS Regression for Quantification of Total Polyphenol Contents from the Fruit and Aerial Parts of Citrullus colocynthis. Phytochem. Anal. 2018, 29, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Fernández, K.; Agosin, E. Quantitative Analysis of Red Wine Tannins Using Fourier-Transform Mid-Infrared Spectrometry. J. Agric. Food Chem. 2007, 55, 7294–7300. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.M.; Oliveira, L.S.; Franca, A.S. Polyphenols in Jabuticaba (Plinia spp.) Peel Flours: Extraction and Comparative Evaluation of FTIR and HPLC for Quantification of Individual Compounds. Foods 2023, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT-Food Sci. Technol. 2022, 154, 112740. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Schwartz, B.; Peri, I.; Shimoni, E. Improving Bioavailability and Stability of Genistein by Complexation with High-Amylose Corn Starch. J. Agric. Food Chem. 2011, 59, 7932–7938. [Google Scholar] [CrossRef]
- Cohen, R.; Orlova, Y.; Kovalev, M.; Ungar, Y.; Shimoni, E. Structural and functional properties of amylose complexes with genistein. J. Agric. Food Chem. 2008, 56, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhu, J.; Liu, P.; Cui, B.; Abd El-Aty, A.M. Preparation and characterization of octenyl succinylated starch microgels via a water-in-oil (W/O) inverse microemulsion process for loading and releasing epigallocatechin gallate. Food Chem. 2021, 355, 129661. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, Y.-H.; Choi, S.-J.; Rho, S.-J.; Kim, Y.-R. Improving the stability and curcumin retention rate of curcumin-loaded filled hydrogel prepared using 4αGTase-treated rice starch. Foods 2021, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl 2), S33–S50. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Jiang, H.; Zhang, T.; Li, X. Interaction mechanism between green tea extract and human α-amylase for reducing starch digestion. Food Chem. 2015, 186, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT-Food Sci. Technol. 2022, 153, 112455. [Google Scholar] [CrossRef]
- Jane, J.-L.; Robyt, J.F. Structure studies of amylose-V complexes and retro-graded amylose by action of alpha amylases, and a new method for preparing amylodextrins. Carbohydr. Res. 1984, 132, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liu, Z.; Chen, L.; Qiu, Z.; Li, T. Effect of starch-catechin interaction on regulation of starch digestibility during hot-extrusion 3D printing: Structural analysis and simulation study. Food Chem. 2022, 393, 133394. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Xu, F.; Chen, L.; Cao, F.; Cui, C. Effect of Catechin on the Retrogradation and Structural Properties of Chestnut Starch. Starch-Stärke 2022, 74, 2200113. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review: Starch retrogradation…. Compr. Rev. Food. Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, L.; Wang, L.; Li, Z.; Qiu, J. Multi-scale structure, rheological and digestive properties of starch isolated from highland barley kernels subjected to different thermal treatments. Food Hydrocoll. 2022, 129, 107630. [Google Scholar] [CrossRef]
- Wu, Y.; Niu, M.; Xu, H. Pasting behaviors, gel rheological properties, and freeze-thaw stability of rice flour and starch modified by green tea polyphenols. LWT-Food Sci. Technol. 2019, 118, 108796. [Google Scholar] [CrossRef]
- Zeng, X.; Zheng, B.; Xiao, G.; Chen, L. Synergistic effect of extrusion and polyphenol molecular interaction on the short/long-term retrogradation properties of chestnut starch. Carbohydr. Polym. 2022, 276, 118731. [Google Scholar] [CrossRef] [PubMed]
- Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Bello-Perez, L.A.; Gonzalez, M.; Reyes, I.; Alvarez-Poblano, L. Supplementing white maize masa with anthocyanins: Effects on masa rheology and on the in vitro digestibility and hardness of tortillas. J. Cereal Sci. 2020, 91, 102883. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Borrelli, G.M.; Giovanniello, V.; Platani, C.; De Vita, P. Production of anthocyanin-enriched flours of durum and soft pigmented wheats by air-classification, as a potential ingredient for functional bread. J. Cereal Sci. 2018, 79, 118–126. [Google Scholar] [CrossRef]
- Wang, R.B.; Li, M.; Brennan, M.A.; Kulasiri, D.; Guo, B.L.; Brennan, C.S. Phenolic Release during In Vitro Digestion of Cold and Hot Extruded Noodles Supplemented with Starch and Phenolic Extracts. Nutrients 2022, 14, 3864. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, M.; Wu, G.; Hui, X.; Tu, J.; Brennan, M.A.; Guo, B.; Brennan, C.S. Inhibition of phenolics on the in vitro digestion of noodles from the view of phenolics release. Int. J. Food Sci. Technol. 2022, 57, 1208–1217. [Google Scholar] [CrossRef]
- Ou, S.J.L.; Yu, J.; Zhou, W.; Liu, M.H. Effects of anthocyanins on bread microstructure, and their combined impact on starch digestibility. Food Chem. 2022, 374, 131744. [Google Scholar] [CrossRef] [PubMed]

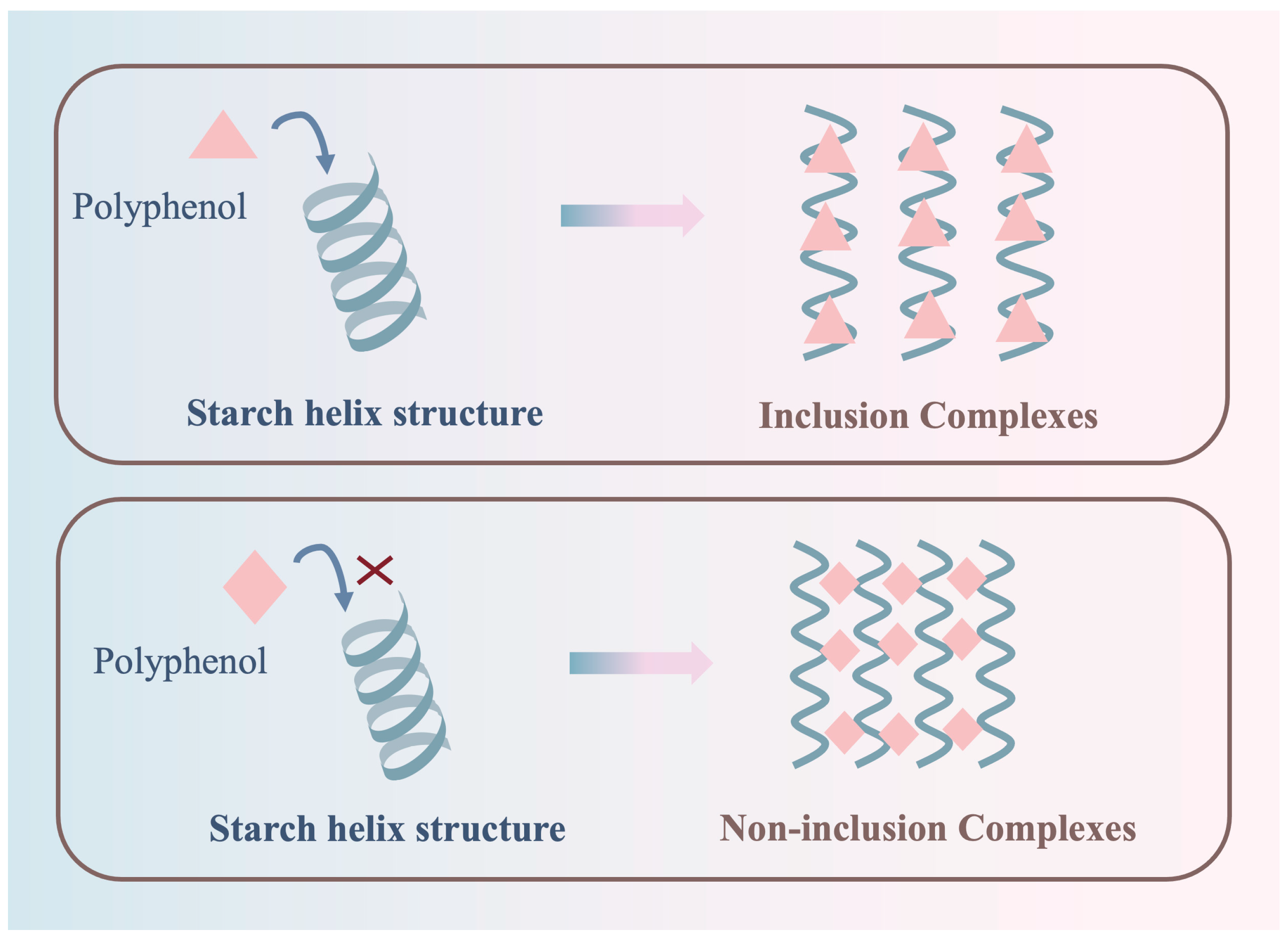
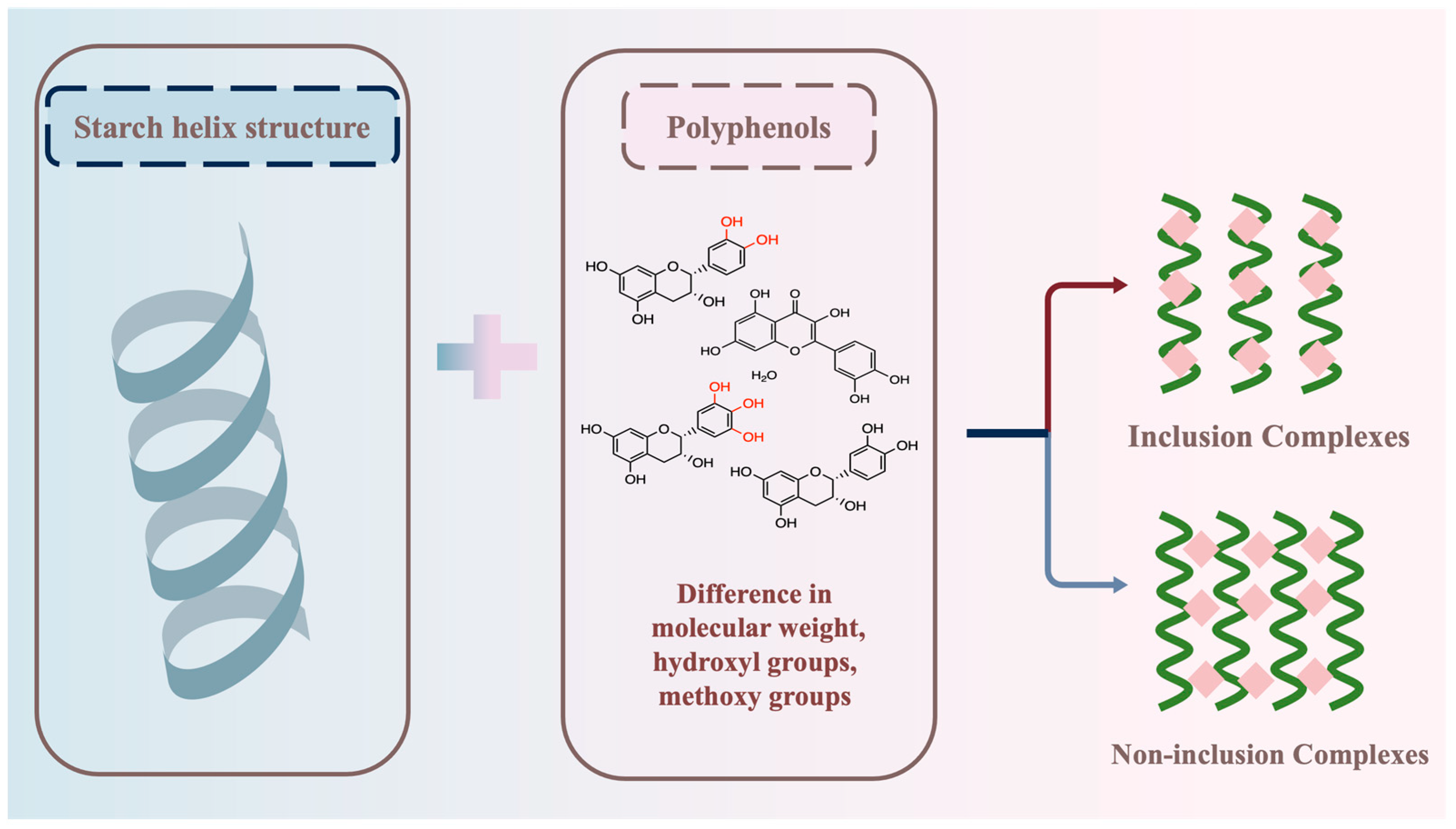


| Starch | Polyphenol | Complex Type | Characterization Techniques | Reference |
|---|---|---|---|---|
| Rice starch | Catechin | V-type | Resistant starch increased α-amylase inhibition | [132] |
| Lotus seed starch | Green tea phenolic | Non-inclusive complexes and V-type inclusion complex | Digestibility lowered | [79] |
| Proso millet starch | Proanthocyanidins | - | Relative crystallinity decreased Solubility and swelling power of starch increased Enthalpy value decreased Resistant starch increased | [47] |
| Potato starch | Dandelion flavonoids | - | Textural parameters decreased Moduli decreased Resistant starch increased Temperatures and enthalpy of gelatinization increased | [48] |
| Wheat starch | Tannic acid | Non-inclusive complexes and V-type inclusion complex | G′ and viscosity increased Digestibility slowed down | [8] |
| Rice starch | Proanthocyanidins from Chinese berry leaves | - | Enthalpy value decreased Thermal stability decreased Digestibility lowered | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liu, Y.; Jia, Y.; Zhang, H.; Ren, F. Formation and Application of Starch–Polyphenol Complexes: Influencing Factors and Rapid Screening Based on Chemometrics. Foods 2024, 13, 1557. https://doi.org/10.3390/foods13101557
Wu Y, Liu Y, Jia Y, Zhang H, Ren F. Formation and Application of Starch–Polyphenol Complexes: Influencing Factors and Rapid Screening Based on Chemometrics. Foods. 2024; 13(10):1557. https://doi.org/10.3390/foods13101557
Chicago/Turabian StyleWu, Yingying, Yanan Liu, Yuanqiang Jia, Huijuan Zhang, and Feiyue Ren. 2024. "Formation and Application of Starch–Polyphenol Complexes: Influencing Factors and Rapid Screening Based on Chemometrics" Foods 13, no. 10: 1557. https://doi.org/10.3390/foods13101557







