Effect of Pod Storage and Drying Temperature on Fermentation Dynamics and Final Bean Quality of Cacao Nacional in Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Set-Up Experiments and Sampling
2.2. Analyses
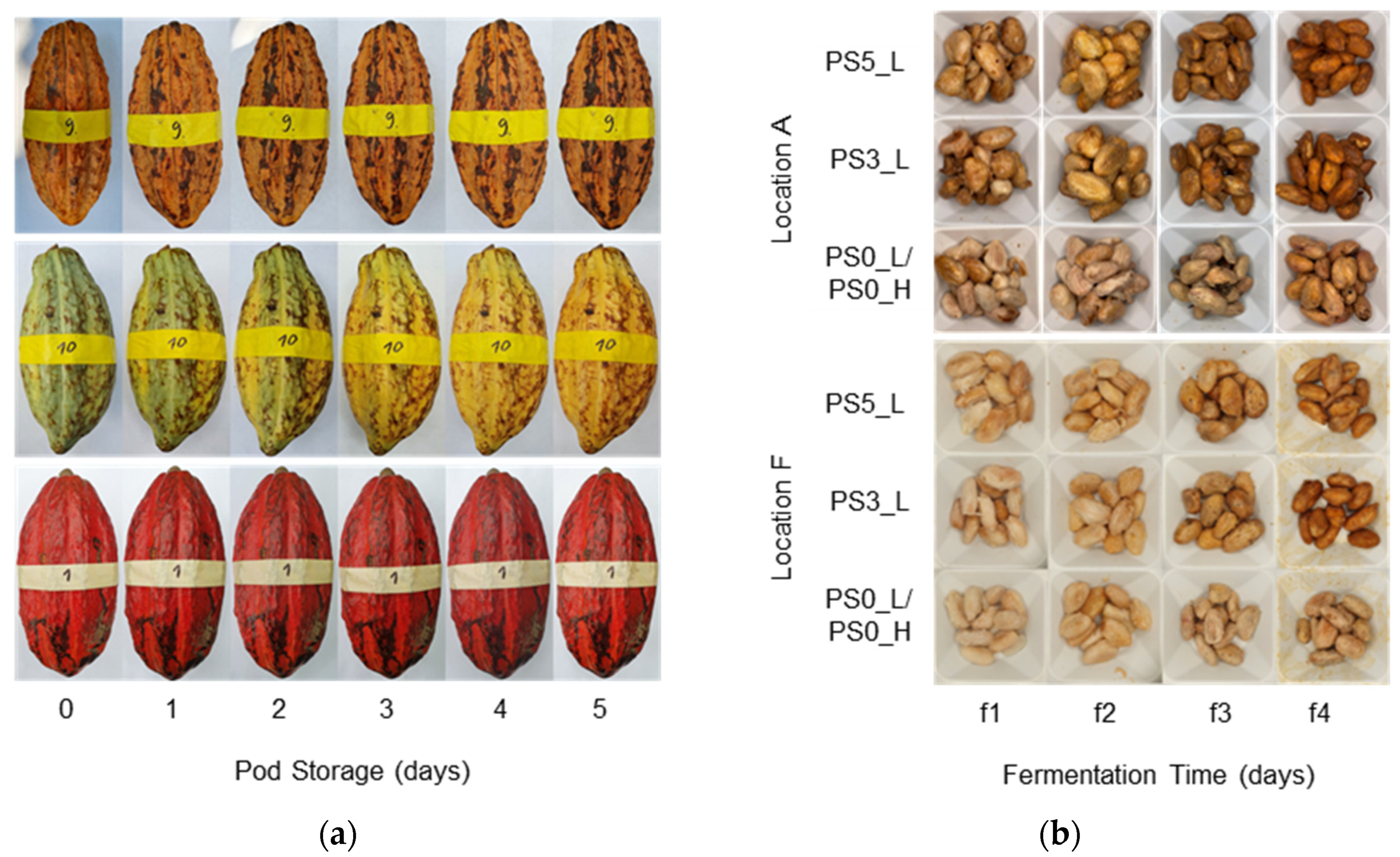
2.2.1. Monitoring of Pod Weight, Pod and Bean Color
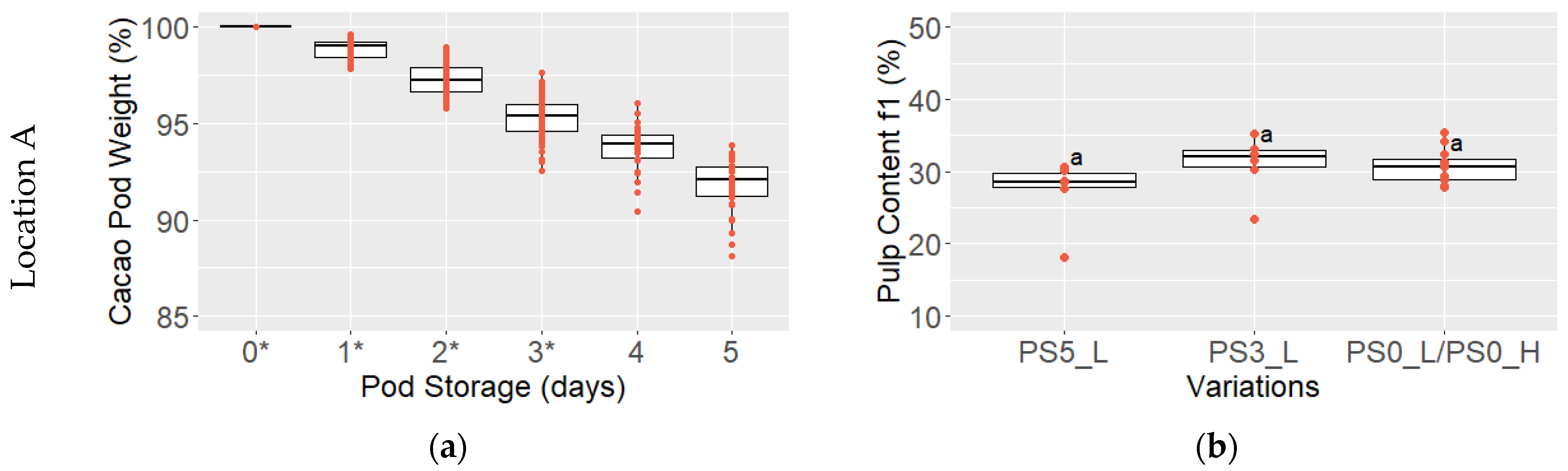
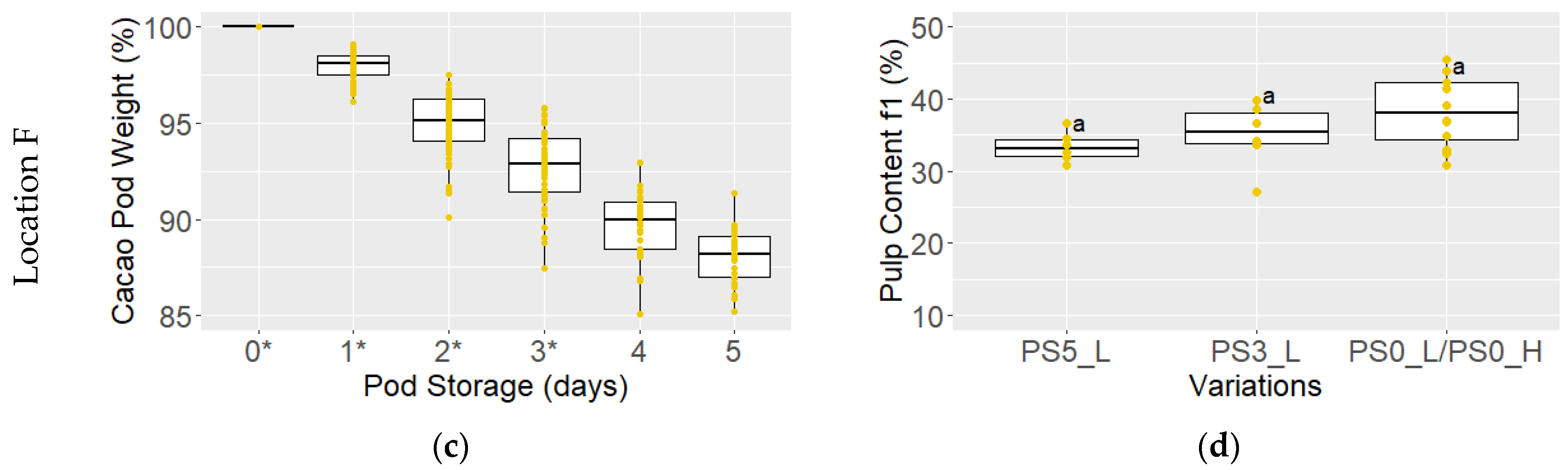
2.2.2. Measurement of Pulp Content, pH of Pulp and Cotyledon
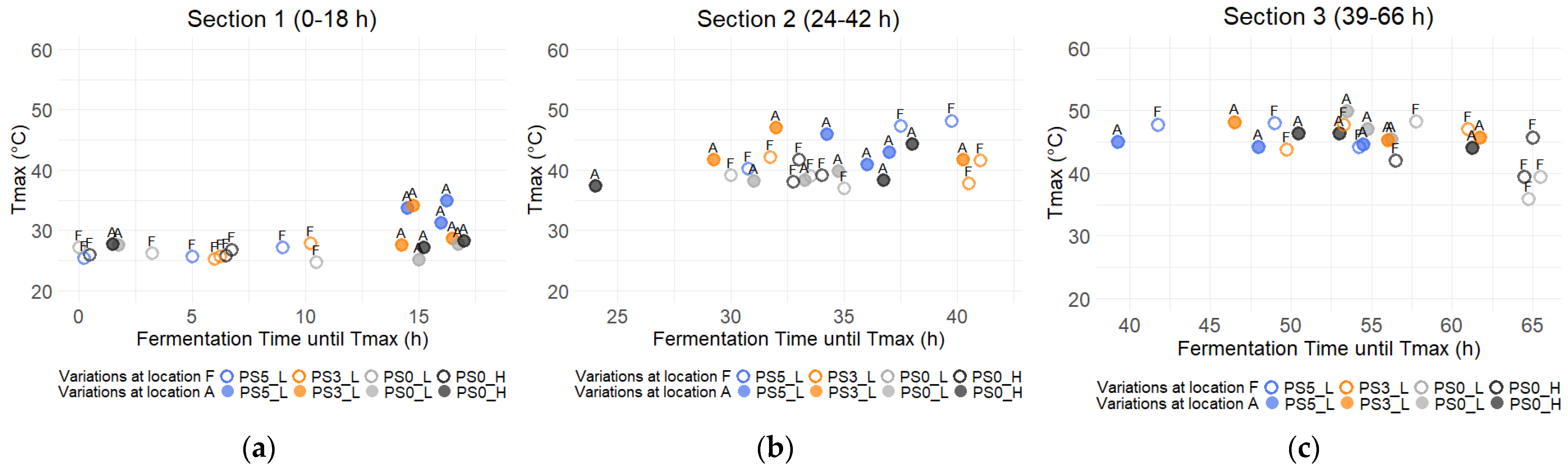
2.2.3. Measurement of Temperature
2.2.4. Enumeration of Yeasts and Lactic Acid Bacteria
2.2.5. Sugars and Organic Acids by HPLC
2.2.6. Cut-Test and Fermentation Index (FI) of Dried Beans
2.2.7. Protein Analysis by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.2.8. Spectrophotometric Determination of Total Free Amino Acids Content
2.2.9. Sensory Evaluation of Cocoa Liquor
2.3. Statistics
3. Results
3.1. Pod and Bean Color, Pod Weight, and Pulp Content
3.2. Yeast and Lactic Acid Bacteria Counts
3.3. Temperature of the Cocoa Bean Mass
3.4. Development of pH of Pulp and Cotyledon
3.5. Sugars and Organic Acids in Dried Beans
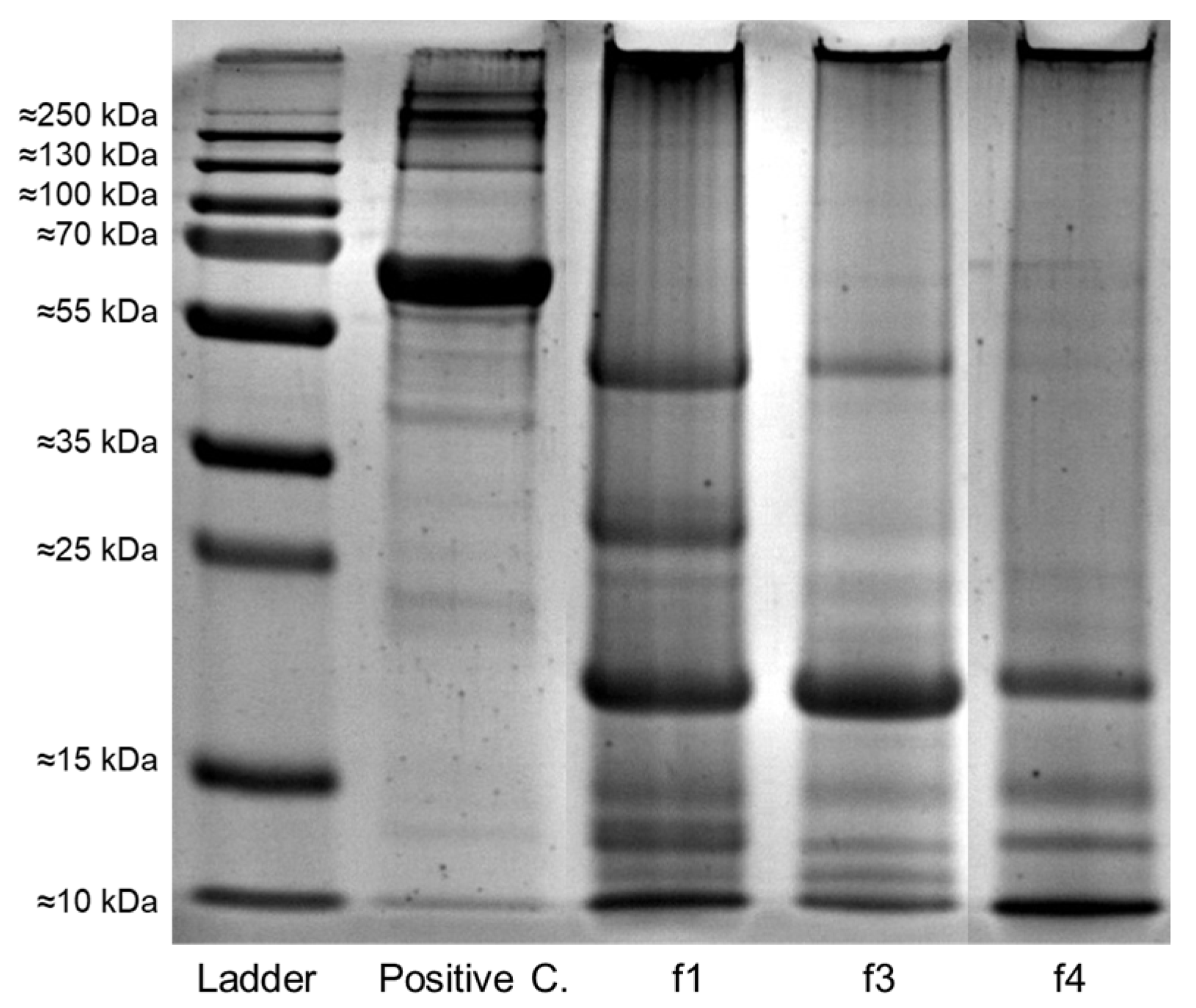
3.6. Protein Profile of Fermenting and Dried Beans
3.7. Content of Total Free Amino Acids
3.8. Fermentation Index and Cut-Test
3.9. Sensory Evaluation of Cocoa Liquor
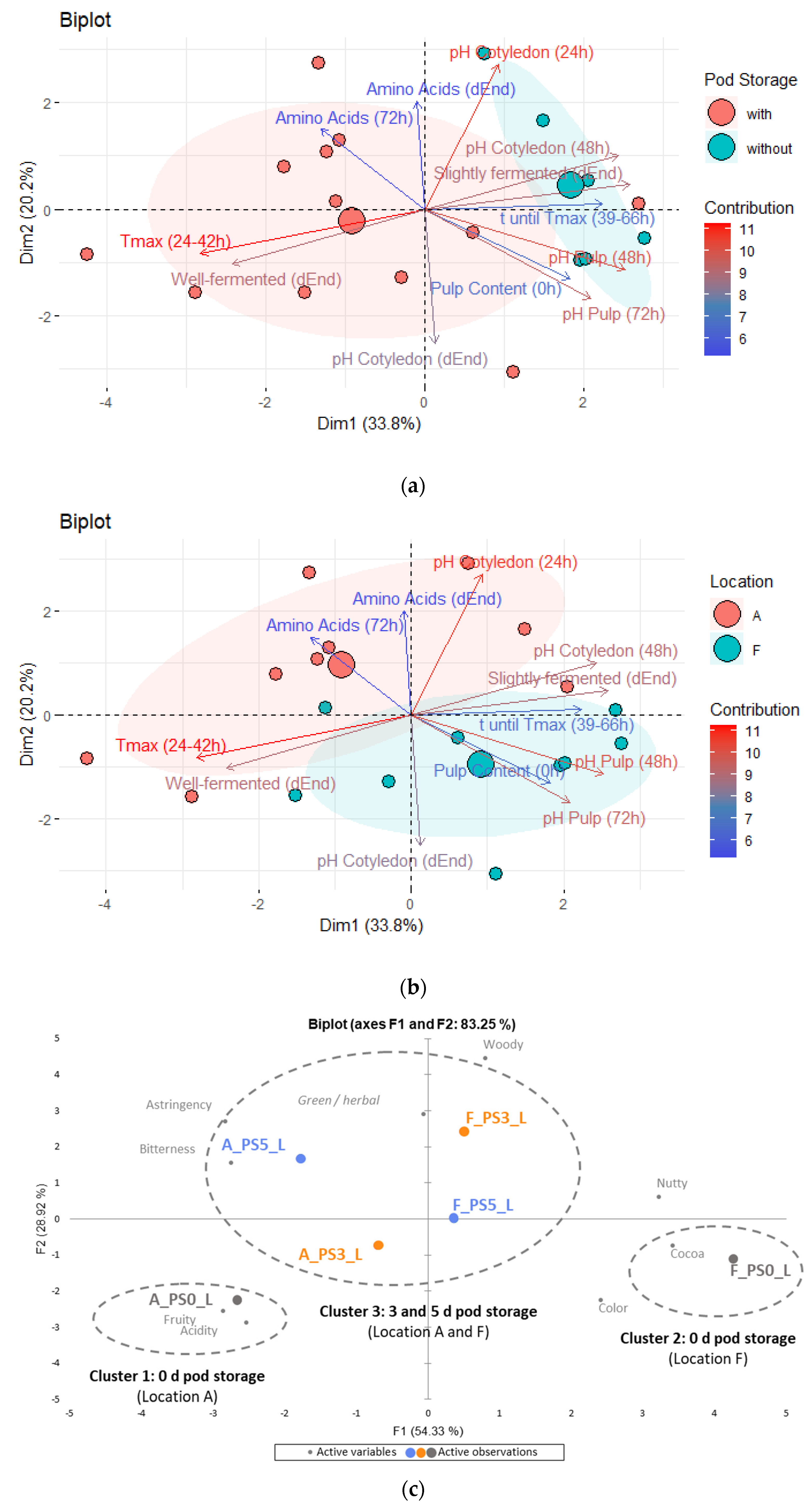
3.10. Principal Component Analysis
3.10.1. Focus on Pod Storage
3.10.2. Focus on Drying Temperatures
4. Discussion
4.1. Influence of Pod Storage on Fermentation and End Quality of Cocoa Beans
4.2. Influence of Drying Temperature on End Quality of Cocoa Beans
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ANECACAO Tipos de Cacao. Available online: https://anecacao.com/cacao-en-el-ecuador/tipos-de-cacao/ (accessed on 28 February 2024).
- ICCO. Fine Flavour Cocoa. Available online: https://www.icco.org/fine-or-flavor-cocoa/ (accessed on 28 February 2024).
- ICOO. Quarterly Bulletin of Cocoa Statistics 22–23 Cocoa Year; International Cocoa Organization: Abidjan, Côte d’Ivoire, 1974. [Google Scholar]
- Villacis, A.; Barrera, V.; Alwang, J.; Caicedo, C.; Quiroz, J.; IDB Invest. Strategies to Strengthen Ecuador’s High-Value Cacao Value Chain; Inter-American Development Bank, 2022. Available online: https://publications.iadb.org/en/node/31654 (accessed on 28 February 2024).
- Perez, M.; Lopez-Yerena, A.; Vallverdú-Queralt, A. Traceability, Authenticity and Sustainability of Cocoa and Chocolate Products: A Challenge for the Chocolate Industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Miescher Schwenninger, S. Monitoring of Cocoa Post-Harvest Process Practices on a Small-Farm Level at Five Locations in Ecuador. Heliyon 2022, 8, e09628. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Djali, M.; Indiarto, R.; Lembong, E.; Baiti, N. Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans. Horticulturae 2023, 9, 805. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M. Diferenciación y Agregado de Valor en la Cadena Ecuatoriana del Cacao; Informes de investigación; Instituto de Altos Estudios Nacionales: Quito, Ecuador, 2015; ISBN 978-9942-950-51-2. [Google Scholar]
- Camu, N.; González, Á.; De Winter, T.; Van Schoor, A.; De Bruyne, K.; Vandamme, P.; Takrama, J.S.; Addo, S.K.; De Vuyst, L. Influence of Turning and Environmental Contamination on the Dynamics of Populations of Lactic Acid and Acetic Acid Bacteria Involved in Spontaneous Cocoa Bean Heap Fermentation in Ghana. Appl. Environ. Microbiol. 2008, 74, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Dulce, V.-R.; Anne, G.; Manuel, K.; Carlos, A.-A.; Jacobo, R.-C.; Sergio De Jesús, C.-E.; Eugenia, L.-C. Cocoa Bean Turning as a Method for Redirecting the Aroma Compound Profile in Artisanal Cocoa Fermentation. Heliyon 2021, 7, e07694. [Google Scholar] [CrossRef] [PubMed]
- Guehi, T.S.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, D.K.; Zahouli, I.B. Effect of Turning Beans and Fermentation Method on the Acidity and Physical Quality of Raw Cocoa Beans. Adv. J. Food Sci. Technol. 2010, 2, 163–171. [Google Scholar]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of Turning, Pod Storage and Fermentation Time on Microbial Ecology and Volatile Composition of Cocoa Beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Mbonomo, R.B.; Medap, A.S.Z.; Brecht, J.K.; Eyame, G. A Study of the Combined Effect of Post-Harvest Fermentation, Turning and Drying of Cocoa (Theobroma cacao L.) on Beans Quality. J. Multidiscipl. Eng. Sci. Technol. (JMEST) 2016, 3, 5. [Google Scholar]
- Hashim, P.; Selamat, J.; Muhammad, S.K.S.; Ali, A. Changes in Free Amino Acid, Peptide-N, Sugar and Pyrazine Concentration during Cocoa Fermentation. J. Sci. Food Agric. 1998, 78, 543–550. [Google Scholar] [CrossRef]
- Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Motzer, D.; Poulose-Züst, M.; Miescher Schwenninger, S. Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory. Foods 2024, 13, 137. [Google Scholar] [CrossRef]
- Liendo, R.J. Efecto del volteo sobre los perfiles sensoriales del cacao fermentado. Rev. Fac. Agron. 2015, 32, 41–62. [Google Scholar]
- Ackah, E.; Dompey, E. Effects of Fermentation and Drying Durations on the Quality of Cocoa (Theobroma cacao L.) Beans during the Rainy Season in the Juaboso District of the Western-North Region, Ghana. Bull. Natl. Res. Cent. 2021, 45, 175. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of Starter Cultures and Fermentation Techniques on the Volatile Aroma and Sensory Profile of Chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Kongor, J.E.; Takrama, J.F.; Budu, A.S.; Mensah-Brown, H.; Afoakwa, O. Effects of Fermentation and Drying on the Fermentation Index and Cut Test of Pulp Pre-Conditioned Ghanaian Cocoa (Theobroma cacao) Beans. J. Food Sci. Eng. 2013, 625–634. Available online: https://www.researchgate.net/publication/270272357 (accessed on 28 February 2024).
- Díaz-Muñoz, C.; De Vuyst, L. Functional Yeast Starter Cultures for Cocoa Fermentation. J. Appl. Microbiol. 2022, 133, 39–66. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Muñoz, C.; Van De Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Curing of Cocoa Beans: Fine-Scale Monitoring of the Starter Cultures Applied and Metabolomics of the Fermentation and Drying Steps. Front. Microbiol. 2021, 11, 616875. [Google Scholar] [CrossRef]
- Romanens, E.; Pedan, V.; Meile, L.; Miescher Schwenninger, S. Influence of Two Anti-Fungal Lactobacillus Fermentum-Saccharomyces Cerevisiae Co-Cultures on Cocoa Bean Fermentation and Final Bean Quality. PLoS ONE 2020, 15, e0239365. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, M.V.S.; Yallappa, B.S.; Varadaraj, M.C.; Puranaik, J.; Rao, L.J.; Janardhan, P.; Murthy, P.S. Inoculum of the Starter Consortia and Interactive Metabolic Process in Enhancing Quality of Cocoa Bean (Theobroma cacao) Fermentation. LWT—Food Sci. Technol. 2016, 65, 731–738. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Assi-Clair, B.J.; Koné, M.K.; Kouamé, K.; Lahon, M.C.; Berthiot, L.; Durand, N.; Lebrun, M.; Julien-Ortiz, A.; Maraval, I.; Boulanger, R.; et al. Effect of Aroma Potential of Saccharomyces Cerevisiae Fermentation on the Volatile Profile of Raw Cocoa and Sensory Attributes of Chocolate Produced Thereof. Eur. Food Res. Technol. 2019, 245, 1459–1471. [Google Scholar] [CrossRef]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces Cerevisiae and Torulaspora Delbrueckii Starter Cultures on Cocoa Beans Fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef]
- Viesser, J.A.; De Melo Pereira, G.V.; De Carvalho Neto, D.P.; Vandenberghe, L.P.D.S.; Azevedo, V.; Brenig, B.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Exploring the Contribution of Fructophilic Lactic Acid Bacteria to Cocoa Beans Fermentation: Isolation, Selection and Evaluation. Food Res. Int. 2020, 136, 109478. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Wood, G.A.; Lass, R.A. Cocoa, 4th ed.; Blackwell: Oxford, UK, 2008; ISBN 978-0-632-06398-7. [Google Scholar]
- Biehl, B.; Meyer, B.; Crone, G.; Pollmann, L.; Said, M.B. Chemical and Physical Changes in the Pulp during Ripening and Post-Harvest Storage of Cocoa Pods. J. Sci. Food Agric. 1989, 48, 189–208. [Google Scholar] [CrossRef]
- Koné, K.M.; Assi-Clair, B.J.; Kouassi, A.D.D.; Yao, A.K.; Ban-Koffi, L.; Durand, N.; Lebrun, M.; Maraval, I.; Bonlanger, R.; Guehi, T.S. Pod Storage Time and Spontaneous Fermentation Treatments and Their Impact on the Generation of Cocoa Flavour Precursor Compounds. Int. J. Food Sci. Technol. 2021, 56, 2516–2529. [Google Scholar] [CrossRef]
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the Influence of Pod Storage on Sugar and Free Amino Acid Profiles and the Implications on Some Maillard Reaction Related Flavor Volatiles in Forastero Cocoa Beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Budu, A.S.; Takrama, J.; Saalia, F.K. Effect of Pulp Preconditioning on Acidification, Proteolysis, Sugars and Free Fatty Acids Concentration during Fermentation of Cocoa (Theobroma cacao) Beans. Int. J. Food Sci. Nutr. 2011, 62, 755–764. [Google Scholar] [CrossRef]
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef] [PubMed]
- Biehl, B.; Meyer, B.; Said, M.B.; Samarakoddy, R.J. Bean Spreading: A Method for Pulp Preconditioning to Impair Strong Nib Acidification during Cocoa Fermentation in Malaysia. J. Sci. Food Agric. 1990, 51, 35–45. [Google Scholar] [CrossRef]
- Meyer, B.; Biehl, B.; Said, M.B.; Samarakoddy, R.J. Post-harvest Pod Storage: A Method for Pulp Preconditioning to Impair Strong Nib Acidification during Cocoa Fermentation in Malaysia. J. Sci. Food Agric. 1989, 48, 285–304. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts Are Essential for Cocoa Bean Fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Bangerter, U.; Beh, B.H.; Callis, A.B.; Pilkington, I.J. Treatment of Cocoa Beans for Improving Fermentation 1991. European Patent Patent No. 91101882.8, 11 February 1991. [Google Scholar]
- Bickel Haase, T.; Naumann-Gola, S.; Ortner, E.; Zorn, H.; Schweiggert-Weisz, U. Thermal Stabilisation of Cocoa Fruit Pulp—Effects on Sensory Properties, Colour and Microbiological Stability. Curr. Res. Food Sci. 2023, 7, 100549. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O. Cocoa Production and Processing Technology, 1st ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of Volatile and Non-Volatile Compounds in Cocoa (Theobroma cacao L.) during Fermentation and Drying Processes Using Principal Components Analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of Fermentation Time and Drying Temperature on Volatile Compounds in Cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, D. A Comparative Study of Effects of Drying Methods on Quality of Cocoa Beans. Int. J. Eng. Res. 2014, 3, 991–996. [Google Scholar]
- Hii, C.L.; Law, C.L.; Cloke, M.; Suzannah, S. Thin Layer Drying Kinetics of Cocoa and Dried Product Quality. Biosyst. Eng. 2009, 102, 153–161. [Google Scholar] [CrossRef]
- Romanens, E.; Näf, R.; Lobmaier, T.; Pedan, V.; Leischtfeld, S.F.; Meile, L.; Schwenninger, S.M. A Lab-Scale Model System for Cocoa Bean Fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 3349–3362. [Google Scholar] [CrossRef] [PubMed]
- ISO 2451:2017; Cocoa Beans Specification and Quality Requirements. International Organization for Standardization: Vernier, Switzerland, 2017.
- Gourieva, K.B.; Tserrevitinov, O.B. Method of Evaluating the Degree of Fermentation of Cocoa Beans 1979. U.S Patent, 64654, 5 February 1979. [Google Scholar]
- Hansen, C.E.; Olmo, M.D.; Burri, C. Enzyme Activities in Cocoa Beans during Fermentation. J. Sci. Food Agric. 1998, 77, 273–281. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using O-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins1. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Murthy, M.V.R.; Padmanabhan, S.; Ramakrishna, M.; Lonsane, B.K. Comparison of Nine Different Caseinolytic Assays for Estimation of Proteinase Activity and Further Improvement of the Best Method. Food Biotechnol. 1997, 11, 1–23. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L.; Taylor, S. Sensory Evaluation Practice, 2nd ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Paul, V.; Pandey, R. Role of Internal Atmosphere on Fruit Ripening and Storability—A Review. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef] [PubMed]
- Bariah, S.K.; Tajul, A.Y. Effect of Cocoa Pods Storage on the Temperature and Physicochemical Changes during Shallow Box Fermentation. IJISET Int. J. Innov. Sci. Eng. Technol. 2017, 4, 197–203. [Google Scholar]
- Hue, C.; Gunata, Z.; Breysse, A.; Davrieux, F.; Boulanger, R.; Sauvage, F.X. Impact of Fermentation on Nitrogenous Compounds of Cocoa Beans (Theobroma cacao L.) from Various Origins. Food Chem. 2016, 192, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Kofi, K.J.; Grimbs, S.; D’Souza, R.N.; Kuhnert, N.; Vrancken, G.; Ullrich, M.S. Biochemical Fate of Vicilin Storage Protein during Fermentation and Drying of Cocoa Beans. Food Res. Int. 2016, 90, 53–65. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.N.; Grimbs, A.; Grimbs, S.; Behrends, B.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Degradation of Cocoa Proteins into Oligopeptides during Spontaneous Fermentation of Cocoa Beans. Food Res. Int. 2018, 109, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, M.; Abotsi, E.E.; Van de Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Simonis, J.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; et al. Pod Storage with Roasting: A Tool to Diversifying the Flavor Profiles of Dark Chocolates Produced from ‘Bulk’ Cocoa Beans? (Part I: Aroma Profiling of Chocolates). Food Res. Int. 2019, 119, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Jennifer, Q.; Agnes, S.B.; Jemmy, S.T.; Saalia, F. Influence of Pulp-Preconditioning and Fermentation on Fermentative Quality and Appearance of Ghanaian Cocoa (Theobroma cacao) Beans.Pdf. Int. Food Res. J. 2012, 19, 127–133. [Google Scholar]
- Mounjouenpou, P.; Gueule, D.; Tetmoun, S.A.M.; Guyot, B.; Fontana-Tachon, A.; Guiraud, J.P. Incidence of Pod Integrity on the Fungal Microflora and Ochratoxin-A Production in Cocoa. J. Biol. Life Sci. 2012, 3, 254–265. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology; Wiley-Blackwell: Chichester, UK, 2010; ISBN 978-1-4051-9906-3. [Google Scholar]
- Nazaruddin, R.; Seng, L.K.; Hassan, O.; Said, M. Effect of Pulp Preconditioning on the Content of Polyphenols in Cocoa Beans (Theobroma cacao) during Fermentation. Ind. Crops Prod. 2006, 24, 87–94. [Google Scholar] [CrossRef]
- Lima, L.J.R.; Nout, M.J.R. Quality and Safety of Cocoa Beans. In Cocoa and Coffee Fermentations; Schwan, R.F., Fleet, G.H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 227–269. [Google Scholar]
- Voigt, J.; Lieberei, R. Biochemistry of Cocoa Fermentation. In Cocoa and Coffee Fermentations; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2015; pp. 193–225. [Google Scholar]
- Borthwick, A.D.; Da Costa, N.C. 2,5-Diketopiperazines in Food and Beverages: Taste and Bioactivity. Crit. Rev. Food Sci. Nutr. 2017, 57, 718–742. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors Influencing Quality Variation in Cocoa (Theobroma cacao) Bean Flavour Profile—A Review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- André, A.; Casty, B.; Ullrich, L.; Chetschik, I. Use of Molecular Networking to Identify 2,5-Diketopiperazines in Chocolates as Potential Markers of Bean Variety. Heliyon 2022, 8, e10770. [Google Scholar] [CrossRef]
- Macedo, A.S.L.; Rocha, F.D.S.; Ribeiro, M.D.S.; Soares, S.E.; Bispo, E.D.S. Characterization of Polyphenol Oxidase in Two Cocoa (Theobroma cacao L.) Cultivars Produced in the South of Bahia, Brazil. Food Sci. Technol. 2016, 36, 56–63. [Google Scholar] [CrossRef]
- Jinap, S.; Thien, J.; Yap, T.N. Effect of Drying on Acidity and Volatile Fatty Acids Content of Cocoa Beans. J. Sci. Food Agric. 1994, 65, 67–75. [Google Scholar] [CrossRef]
- Amoa-Awua, W.K. Methods of Cocoa Fermentation and Drying. In Cocoa and Coffee Fermentations; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2015. [Google Scholar]








| Category | Attributes | Definition | Scale |
|---|---|---|---|
| Appearance | Color | Color impression on the surface of the liquefied cocoa mass | Light–dark |
| Texture | Viscosity | Flow behavior of the cocoa liquor on the palate | Low–high |
| Finesse | Perception of the size and quantity of particles in the melted cocoa liquor | Coarse–fine | |
| Basic taste | Acidity | Basic taste, perception of the intensity of acidity like a solution of citric acid or acetic acid | Low–intense |
| Bitterness | Basic taste, perception of the intensity of bitterness like a caffein solution | Low–intense | |
| Trigeminal sensation | Astringency | Dry and rough mouthfeel | Low–intense |
| Aroma (retronasal) | Floral | Aroma perception, reminiscent of dried flowers/orange blossom | Low–intense |
| Fruity | Aroma perception, reminiscent of citrus fruits lemon/grapefruit/bergamot | Low–intense | |
| Cocoa | Aroma perception, reminiscent of cocoa powder | Low–intense | |
| Nutty | Aroma perception, reminiscent of unroasted walnuts | Low–intense | |
| Woody | Aroma perception, reminiscent of dry wood | Low–intense | |
| Green/herbal | Aroma perception, reminiscent of dried herbs | Low–intense |
| Location | Variation | Pulp pH | Cotyledon pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | dEnd | ||
| A | PS5_L | 3.9 ± 0.1 a | 3.3 ± 0.3 a | 3.4 ± 0.2 a | 3.6 ± 0.2 a | 6.5 ± 0.1 a | 6.3 ± 0.1 a | 5.5 ± 0.6 a | 5.1 ± 0.3 a | 5.2 ± 0.1 a |
| PS3_L | 4.0 ± 0.2 a | 3.5 ± 0.4 a | 3.6 ± 0.3 a | 3.8 ± 0.3 a | 6.5 ± 0.1 a | 6.3 ± 0.2 a | 5.7 ± 0.7 a | 5.1 ± 0.2 a | 5.3 ± 0.1 a | |
| PS0_L | 3.8 ± 0.1 a | 3.5 ± 0.3 a | 3.9 ± 0.3 a | 4.0 ± 0.2 a | 6.5 ± 0.2 a | 6.5 ± 0.1 a | 6.0 ± 0.4 a | 5.1 ± 0.5 a | 5.0 ± 0.2 a | |
| PS0_H | 5.3 ± 0.4 a | |||||||||
| F | PS5_L | 3.8 ± 0.2 a | 3.5 ± 0.3 a | 3.8 ± 0.1 a | 4.0 ± 0.1 a | 6.3 ± 0.1 a | 6.2 ± 0.1 a | 5.9 ± 0.5 a | 5.8 ± 0.6 a | 5.6 ± 0.3 a |
| PS3_L | 3.8 ± 0.1 a | 3.6 ± 0.3 a | 4.0 ± 0.1 a | 4.3 ± 0.3 a | 6.3 ± 0.1 a | 6.3 ± 0.2 a | 6.0 ± 0.1 a | 5.5 ± 0.4 a | 5.5 ± 0.3 a | |
| PS0_L | 3.7 ± 0.1 a | 3.6 ± 0.2 a | 3.9 ± 0.2 a | 4.1 ± 0.2 a | 6.2 ± 0.1 a | 6.2 ± 0.1 a | 6.1 ± 0.1 a | 5.5 ± 0.4 a | 5.4 ± 0.2 a | |
| PS0_H | 5.2 ± 0.2 a | |||||||||
| Location | Variation | Sugars (mg/g) | Organic Acids (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| Sucrose | Fructose | Glucose | Citric Acid | Lactic Acid | Acetic Acid | ||
| A | PS5_L | 2.9 ± 0.3 a | 1.8 ± 0.2 a | 1.2 ± 0.1 ab | 2.6 ± 0.6 a | 6.7 ± 1.1 a | 2.3 ± 1.0 a |
| PS3_L | 2.3 ± 0.8 a | 2.0 ± 0.0 a | 1.4 ± 0.3 a | 2.9 ± 0.4 a | 6.1 ± 0.8 ab | 3.8 ± 1.0 a | |
| PS0_L | 2.3 ± 0.9 a | 1.9 ± 0.5 a | 1.3 ± 0.5 ab | 2.3 ± 0.6 a | 4.9 ± 0.9 ab | 5.5 ± 2.0 a | |
| PS0_H | 3.6 ± 1.4 a | 1.4 ± 0.2 a | 0.5 ± 0.4 b | 2.6 ± 0.8 a | 3.1 ± 1.6 b | 3.5 ± 0.9 a | |
| F | PS5_L | 3.4 ± 0.7 a | 1.8 ± 0.3 a | 0.8 ± 0.0 a | 3.6 ± 0.7 a | 3.9 ± 1.7 a | 4.3 ± 2.0 a |
| PS3_L | 3.7 ± 0.6 a | 1.5 ± 0.1 ab | 0.7 ± 0.3 a | 3.0 ± 0.6 a | 4.5 ± 0.9 a | 5.0 ± 2.9 a | |
| PS0_L | 3.3 ± 0.7 a | 1.5 ± 0.5 ab | 0.8 ± 0.4 a | 3.3 ± 0.3 a | 5.3 ± 0.4 a | 3.6 ± 2.3 a | |
| PS0_H | 4.0 ± 0.3 a | 1.0 ± 0.1 b | 0.4 ± 0.3 a | 3.8 ± 0.3 a | 4.4 ± 0.9 a | 3.3 ± 1.6 a | |
| Location | Variation | FermentationIndex | Cut-Test | ||
|---|---|---|---|---|---|
| Well-Fermented Beans (%) | Slightly Fermented Beans (%) | Violet Beans (%) | |||
| A | PS5_L | 0.8 ± 0.1 a | 53.0 ± 24.6 ab | 34.3 ± 15.3 bc | 12.7 ± 13.4 a |
| PS3_L | 0.7 ± 0.0 a | 66.3 ± 13.6 a | 16.3 ± 4.7 c | 17.3 ± 9.5 a | |
| PS0_L | 0.8 ± 0.1 a | 20.3 ± 4.7 bc | 67.0 ± 10.5 a | 12.7 ± 8.1 a | |
| PS0_H | 0.8 ± 0.1 a | 9.7 ± 8.5 c | 62.3 ± 12.0 ab | 28.0 ± 20.3 a | |
| F | PS5_L | 0.7 ± 0.1 a | 50.7 ± 30.7 a | 40.0 ± 18.1 b | 9.3 ± 12.9 a |
| PS3_L | 0.7 ± 0.0 a | 39.0 ± 22.5 a | 49.0 ± 3.5 b | 12.0 ± 19.1 a | |
| PS0_L | 0.7 ± 0.1 a | 24.7 ± 13.4 a | 58.3 ± 6.7 b | 17.0 ± 17.0 a | |
| PS0_H | 0.7 ± 0.0 a | 1.7 ± 2.1 a | 93.3 ± 3.2 a | 5.0 ± 3.0 a | |
| Category | Attributes | p-Values | Location A | Location F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PS5_L | PS3_L | PS0_L | PS0_H | PS5_L | PS3_L | PS0_L | PS0_H | |||
| Appearance | Color | <0.05 | 4.7 cd | 4.4 d | 5.3 c | 6.1 b | 5.0 cd | 4.8 cd | 6.2 b | 7.6 a |
| Texture | Viscosity | <0.05 | 5.8 a | 3.5 d | 3.7 cd | 4.8 abcd | 4.2 bcd | 4.3 bcd | 4.8 abc | 5.2 ab |
| Finesse | <0.05 | 3.3 c | 7.2 a | 7.3 a | 6.6 a | 6.1 ab | 5.4 b | 7.0 a | 7.1 a | |
| Basic taste | Acidity | <0.05 | 4.2 bcd | 4.7 bc | 6.2 a | 4.0 bcd | 4.8 b | 3.9 cd | 3.6 d | 3.5 d |
| Bitterness | <0.05 | 5.0 abc | 4.4 c | 4.8 bc | 5.6 ab | 4.5 c | 4.6 c | 4.3 c | 5.6 a | |
| Trigeminal sensation | Astringency | <0.05 | 5.2 abc | 4.8 cd | 4.9 cd | 5.8 a | 4.8 cd | 5.0 bcd | 4.3 d | 5.8 ab |
| Aroma (retronasal) | Floral | 0.12 | 3.4 a | 2.6 a | 2.2 a | 2.4 a | 3.0 a | 2.2 a | 2.3 a | 2.5 a |
| Fruity | <0.05 | 2.7 bc | 3.2 b | 4.7 a | 2.1 cd | 2.5 bc | 2.1 cd | 1.6 d | 1.3 d | |
| Cocoa | <0.05 | 4.7 d | 4.9 cd | 4.6 d | 5.6 ab | 5.1 bcd | 5.0 cd | 5.8 a | 5.5 abc | |
| Nutty | <0.05 | 3.2 c | 3.3 c | 3.2 c | 3.9 bc | 4.1 ab | 4.3 ab | 4.8 abc | 4.2 ab | |
| Woody | <0.05 | 3.5 ab | 2.9 abc | 1.9 c | 3.0 ab | 2.7 bc | 3.8 a | 2.8 abc | 3.3 ab | |
| Green/herbal | <0.05 | 2.2 c | 2.0 c | 2.2 c | 3.4 ab | 2.3 bc | 2.0 bc | 2.1 c | 3.8 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streule, S.; Freimüller Leischtfeld, S.; Chatelain, K.; Miescher Schwenninger, S. Effect of Pod Storage and Drying Temperature on Fermentation Dynamics and Final Bean Quality of Cacao Nacional in Ecuador. Foods 2024, 13, 1536. https://doi.org/10.3390/foods13101536
Streule S, Freimüller Leischtfeld S, Chatelain K, Miescher Schwenninger S. Effect of Pod Storage and Drying Temperature on Fermentation Dynamics and Final Bean Quality of Cacao Nacional in Ecuador. Foods. 2024; 13(10):1536. https://doi.org/10.3390/foods13101536
Chicago/Turabian StyleStreule, Stefanie, Susette Freimüller Leischtfeld, Karin Chatelain, and Susanne Miescher Schwenninger. 2024. "Effect of Pod Storage and Drying Temperature on Fermentation Dynamics and Final Bean Quality of Cacao Nacional in Ecuador" Foods 13, no. 10: 1536. https://doi.org/10.3390/foods13101536





