The Effect of the PhoP/PhoQ System on the Regulation of Multi-Stress Adaptation Induced by Acid Stress in Salmonella Typhimurium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Acid Adaptation of WT and ∆phoP Strains
2.3. Determination of Acid, Heat and Salt Stress Tolerance of Adapted/Non-Adapted WT/Δphop Strains
2.4. Determination of Antibiotic Resistance of Adapted/Non-Adapted WT/Δphop Strains
2.5. RNA Extraction and Library Sequencing
2.6. Transcriptome Analysis
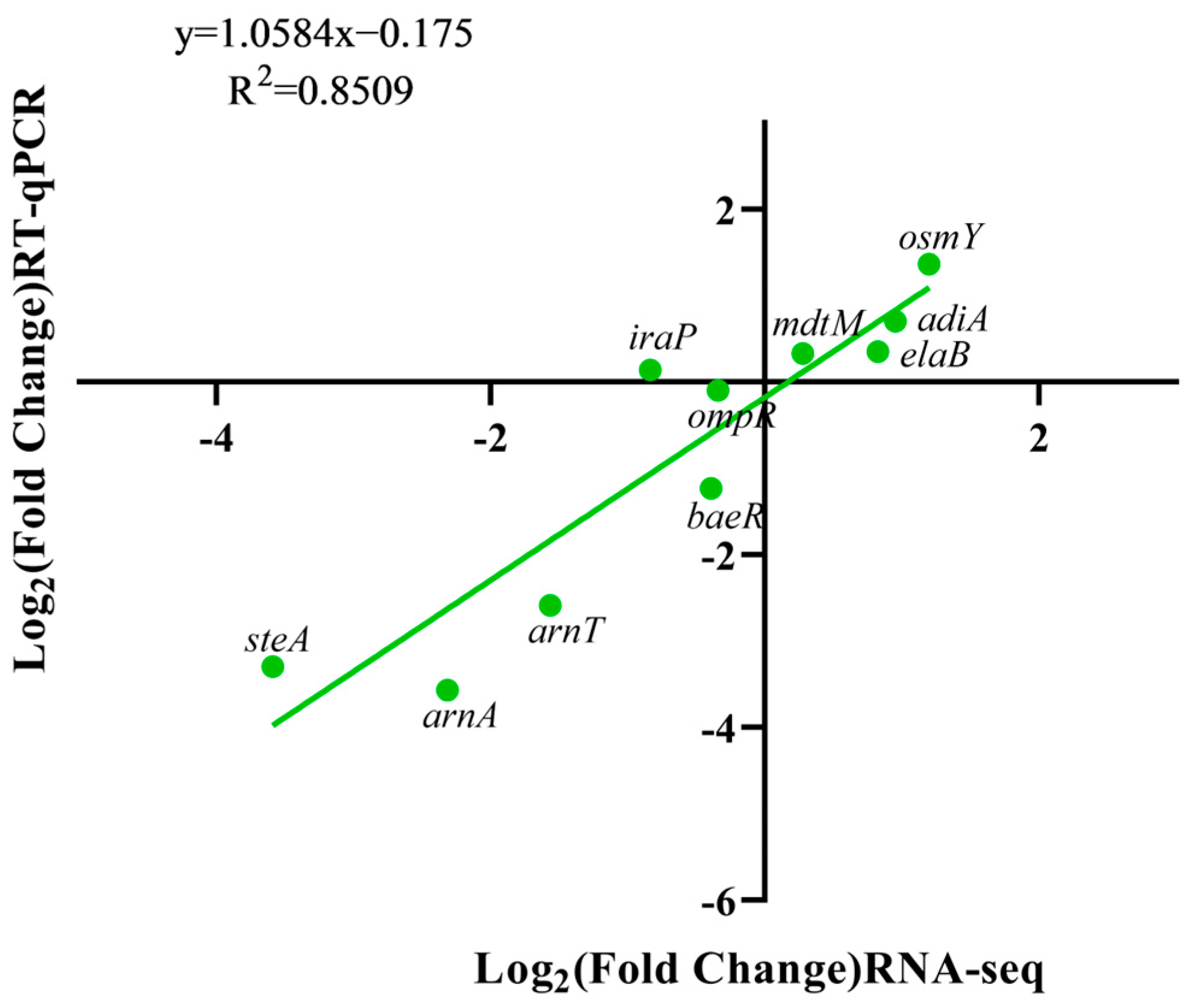
2.7. Determination of Relevant Differential Gene Expression
2.8. Determination of Negative Cell Membrane Surface Charge
2.9. The Persistence of Drug Resistance in the Adapted Salmonella Strains during Low-Temperature Storage in ME
2.10. Statistical Analysis
3. Results
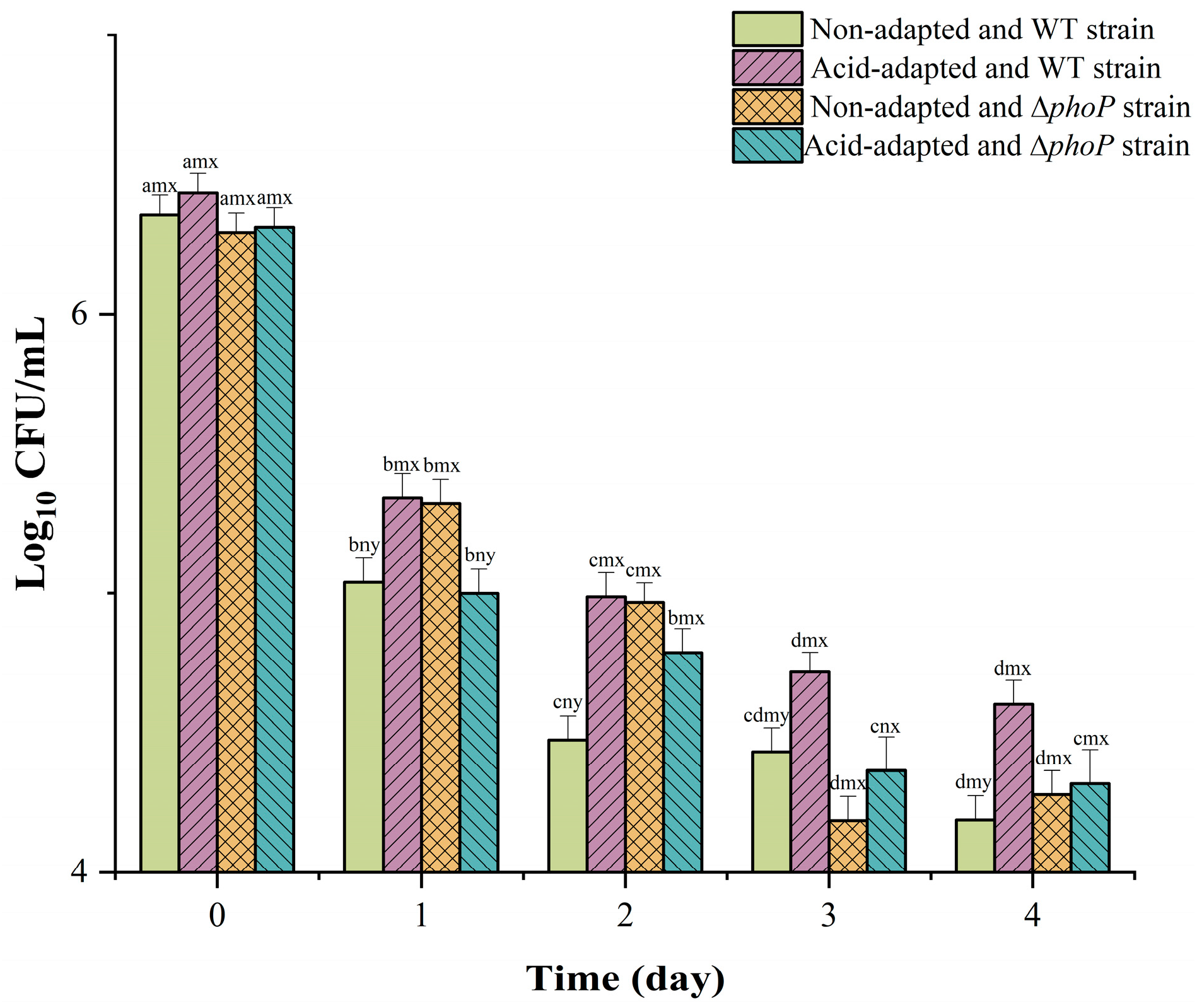
3.1. The Effect of Acid Adaptation and ∆phoP on the Various Resistances of S. typhimurium
3.2. Transcriptomic Analysis
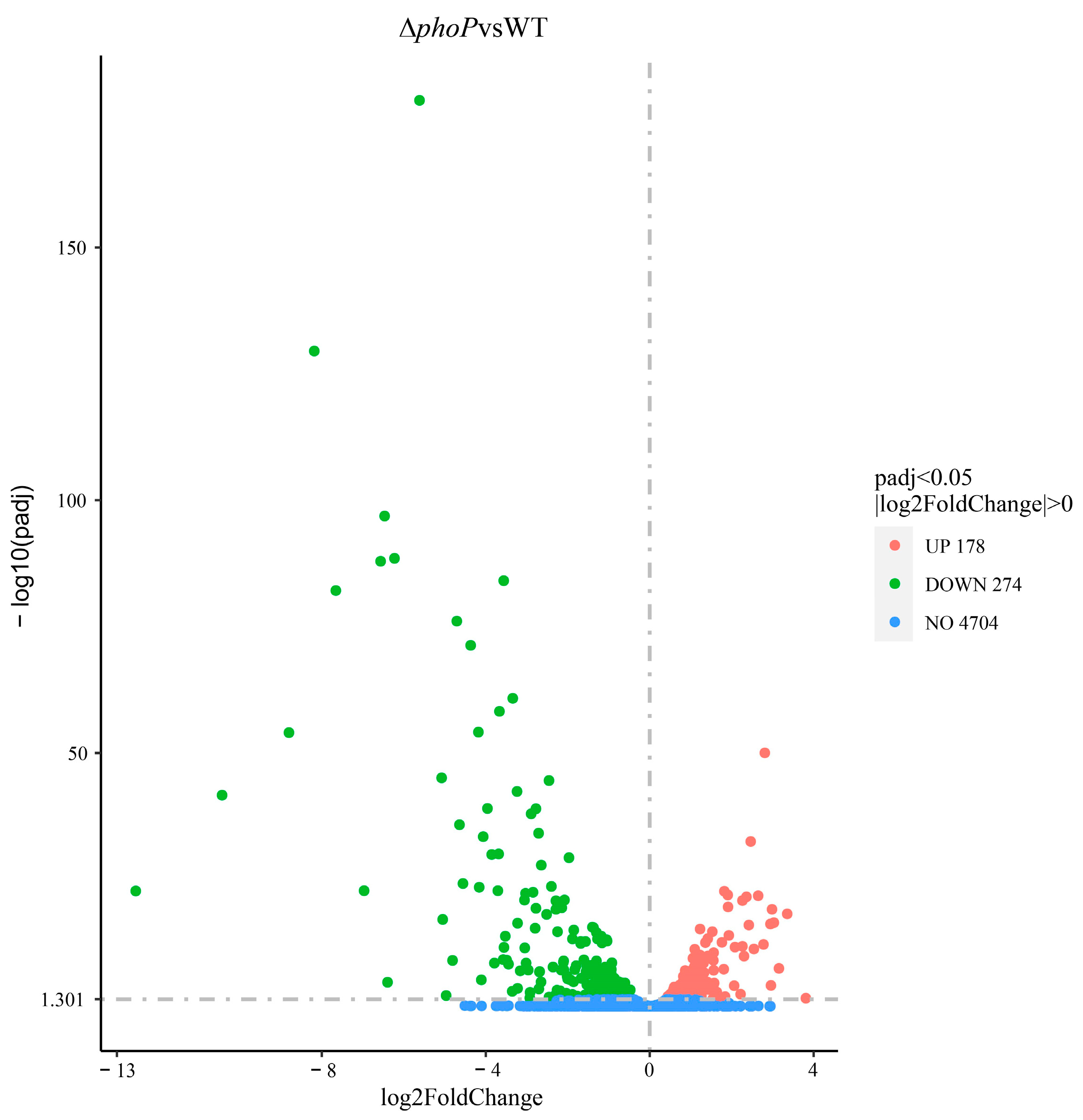
3.2.1. The phoP Gene Plays a Widely Regulated Role in Acid Adaptation
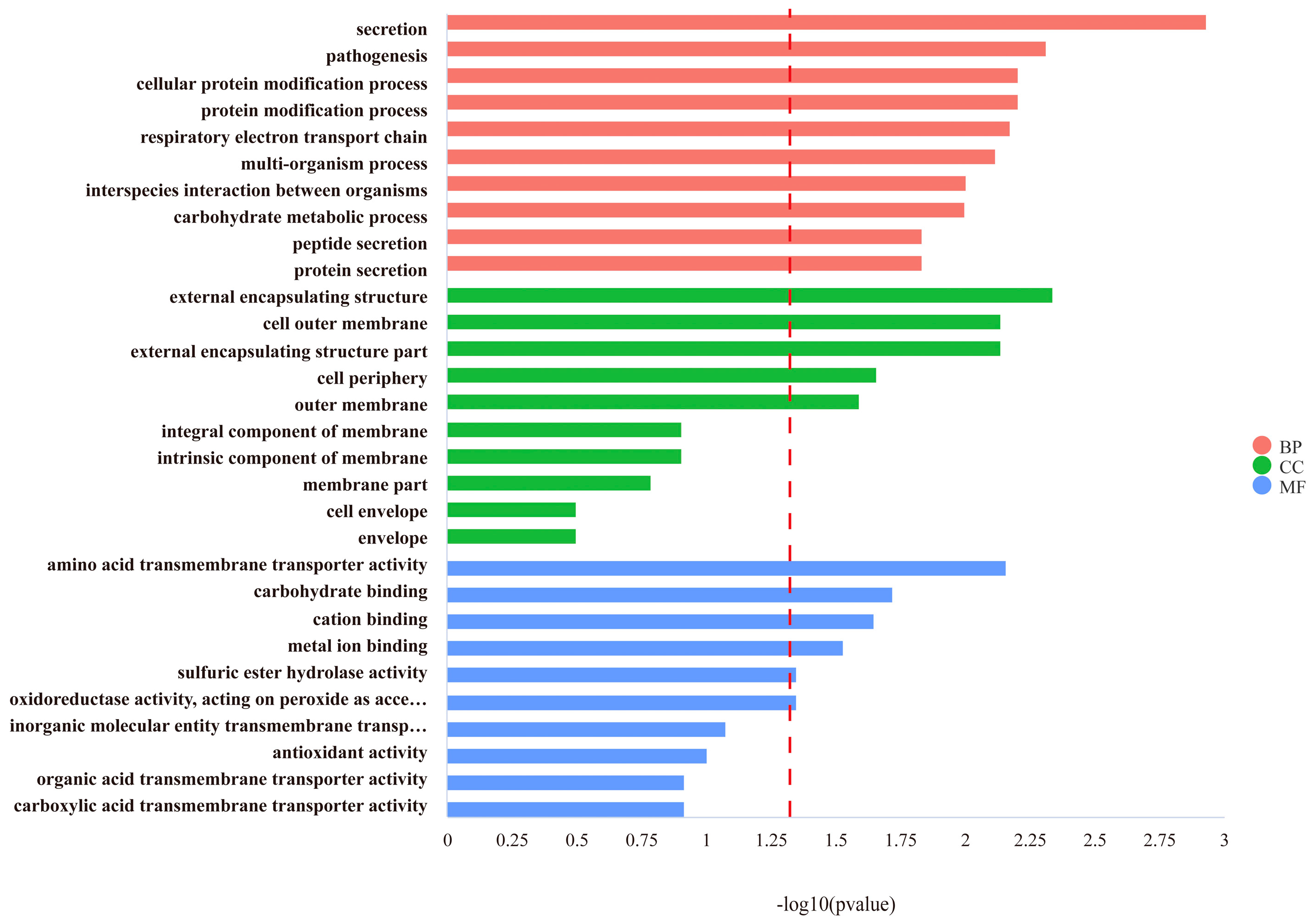
3.2.2. Determining the Function of DEGs by GO and KEGG Pathway Analysis
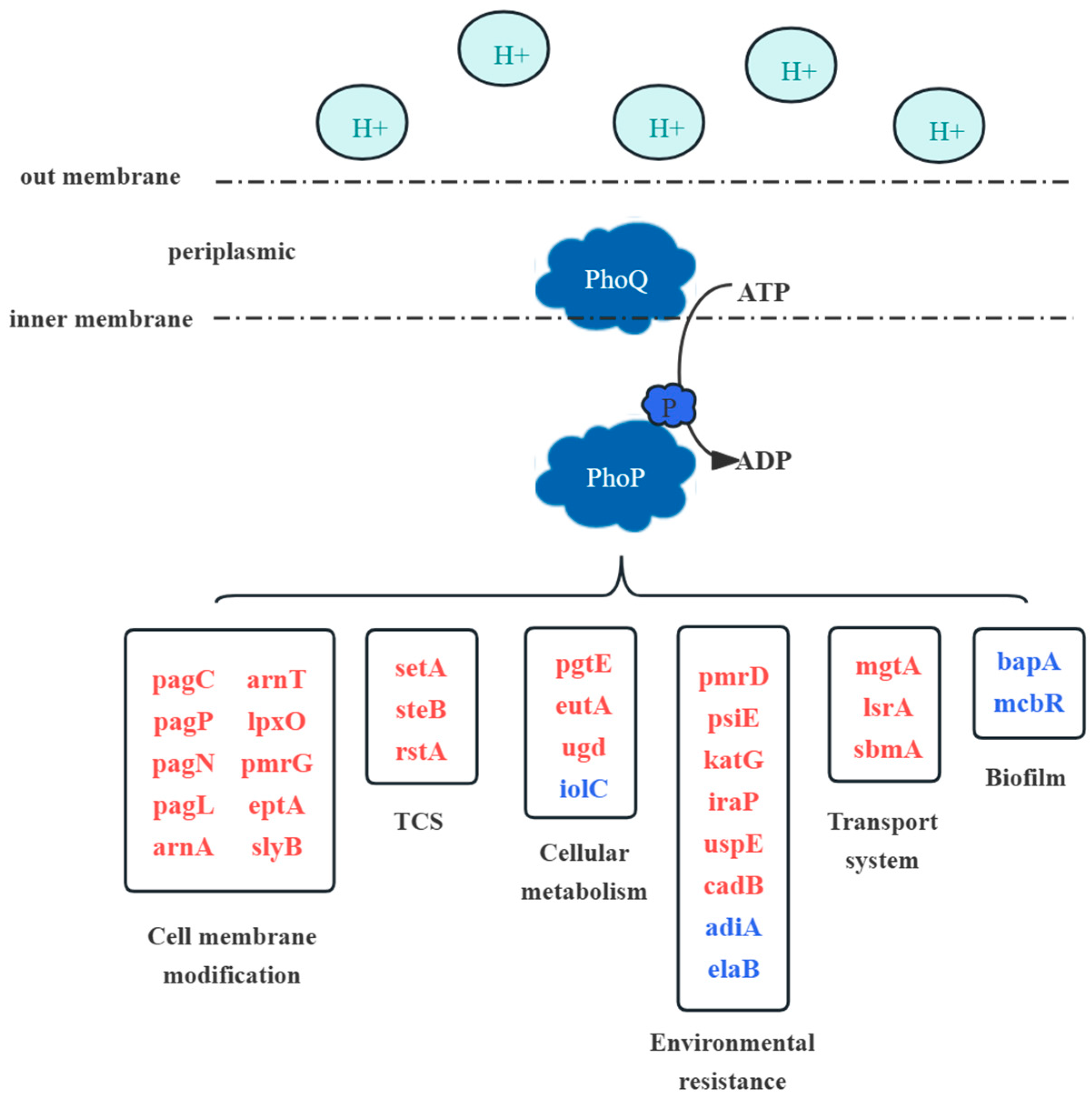
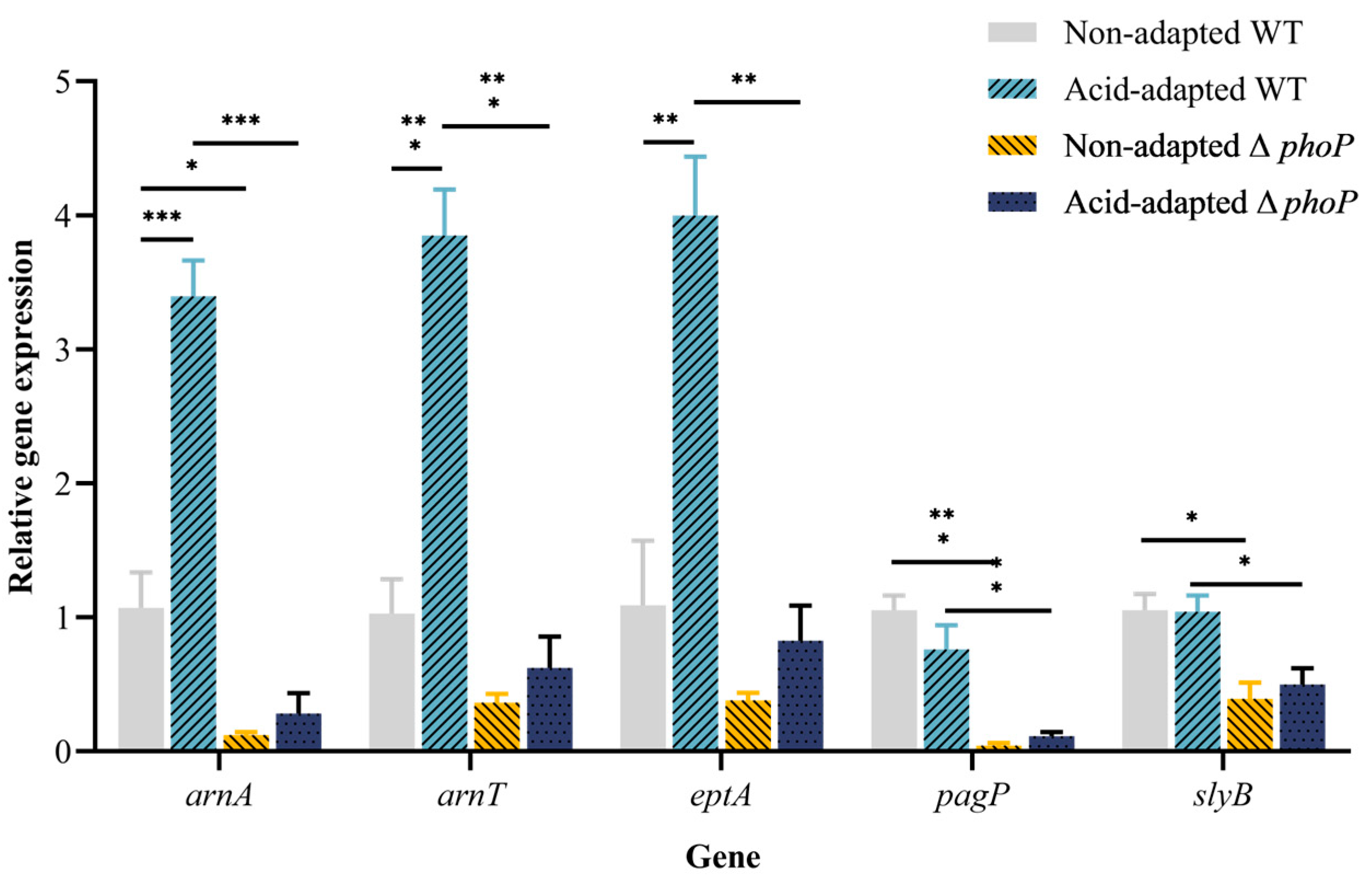
3.3. Antimicrobial Peptide Resistance-Related Gene Expression after Acid Adaptation
3.4. Acid Adaptation and Gene phoP Significantly Affect Salmonella Cell Membrane Surface Charge
3.5. Effect of Acid Adaptation and Gene phoP on Salmonella Drug Resistance during Low-Temperature Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Canning, M.; Birhane, M.G.; Dewey-Mattia, D.; Lawinger, H.; Cote, A.; Gieraltowski, L.; Schwensohn, C.; Tagg, K.A.; Watkins, L.K.F.; Robyn, M.P. Salmonella Outbreaks Linked to Beef, United States, 2012–2019. J. Food Prot. 2023, 86, 100071. [Google Scholar] [CrossRef]
- Brashears, M.M.; Chaves, B.D. The diversity of beef safety: A global reason to strengthen our current systems. Meat Sci. 2017, 132, 59–71. [Google Scholar] [CrossRef]
- Samelis, J.; Sofos, J.N.; Kendall, P.A.; Smith, G.C. Effect of acid adaptation on survival of Escherichia coli O157: H7 in meat decontamination washing fluids and potential effects of organic acid interventions on the microbial ecology of the meat plant environment. J. Food Prot. 2002, 65, 33–40. [Google Scholar] [CrossRef]
- Rossi, G.A.M.; Link, D.T.; Bertolini, A.B.; Tobias, F.L.; Mioni, M.d.S.R. A descriptive review of the use of organic acids and peracetic acid as a decontaminating strategy for meat. eFood 2023, 4, e104. [Google Scholar] [CrossRef]
- Jeremiah, L.; Dugan, M.; Aalhus, J.; Gibson, L. Assessment of the chemical and cooking properties of the major beef muscles and muscle groups. Meat Sci. 2003, 65, 985–992. [Google Scholar] [CrossRef]
- Foster, J.W.; Hall, H.K. Adaptive acidification tolerance response of Salmonella Typhimurium. J. Bacteriol. 1990, 172, 771–778. [Google Scholar] [CrossRef]
- Ye, B.N.; He, S.K.; Zhou, X.J.; Cui, Y.; Zhou, M.; Shi, X.M. Response to Acid Adaptation in Salmonella enterica Serovar Enteritidis. J. Food Sci. 2019, 84, 599–605. [Google Scholar] [CrossRef]
- Liao, X.Y.; Ma, Y.N.; Daliri, E.B.M.; Koseki, S.; Wei, S.; Liu, D.H.; Ye, X.Q.; Chen, S.G.; Ding, T. Interplay of antibiotic resistance and food-associated stress tolerance in foodborne pathogens. Trends Food Sci. Technol. 2020, 95, 97–106. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Shaker, R.R.; Olaimat, A.N.; Jaradat, Z.W.; Elabedeen, N.A.Z.; Holley, R.A. Effects of osmotic pressure, acid, or cold stresses on antibiotic susceptibility of Listeria monocytogenes. Food Microbiol. 2015, 46, 154–160. [Google Scholar] [CrossRef]
- Ebinesh, A.; Vijaykumar, G.; Kiran, T. Exposure to stress minimizes the zone of antimicrobial action: A phenotypic demonstration with six Acinetobacter baumannii strains. MicroMedicine 2018, 6, 16–35. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Elabedeen, N.A.Z.; Jaradat, Z.W.; Shaker, R.R.; Kheirallah, K.A.; Tarazi, Y.H.; Holley, R.A. Impact of environmental stress desiccation, acidity, alkalinity, heat or cold on antibiotic susceptibility of Cronobacter sakazakii. Int. J. Food Microbiol. 2011, 146, 137–143. [Google Scholar] [CrossRef]
- De Sales, C.V.; De Melo, A.N.F.; Niedzwiedzka, K.M.; De Souza, E.L.; Schaffner, D.W.; Magnani, M. Changes of Antibiotic Resistance Phenotype in Outbreak-Linked Salmonella enterica Strains after Exposure to Human Simulated Gastrointestinal Conditions in Chicken Meat. J. Food Prot. 2018, 81, 1844–1850. [Google Scholar] [CrossRef]
- Blair, J.M.; Zeth, K.; Bavro, V.N.; Sancho-Vaello, E. The role of bacterial transport systems in the removal of host antimicrobial peptides in Gram-negative bacteria. FEMS Microbiol. Rev. 2022, 46, fuac032. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Gouyon, P.-H. Arming the enemy: The evolution of resistance to self-proteins. Microbiology 2003, 149, 1367–1375. [Google Scholar] [CrossRef]
- Perez, J.C.; Groisman, E.A. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol. Microbiol. 2007, 63, 283–293. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.W.; Sun, L.; Kong, Z.; Wu, Q.M.; Wang, Z. Transcriptional analysis reveals the relativity of acid tolerance and antimicrobial peptide resistance of Salmonella. Microb. Pathog. 2019, 136, 103701. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.N.; Nychas, G.-J.E. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef] [PubMed]
- de Pina, L.C.; da Silva, F.S.H.; Galvao, T.C.; Pauer, H.; Ferreira, R.B.R.; Antunes, L.C.M. The role of two-component regulatory systems in environmental sensing and virulence in Salmonella. Crit. Rev. Microbiol 2021, 47, 397–434. [Google Scholar] [CrossRef]
- Prost, L.R.; Daley, M.E.; Le Sage, V.; Bader, M.W.; Le Moual, H.; Klevit, R.E.; Miller, S.I. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 2007, 26, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Groisman, E.A. Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol. Microbiol. 2014, 91, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, Y.; Wang, T.; Sun, J.; Zhang, Y.; Ji, J.; Sun, X. Effects of acid, alkaline, cold, and heat environmental stresses on the antibiotic resistance of the Salmonella enterica serovar Typhimurium. Food Res. Int. 2021, 144, 110359. [Google Scholar] [CrossRef] [PubMed]
- Spector, M.P.; Kenyon, W.J. Resistance and survival strategies of Salmonella enterica to environmental stresses. Food Res. Int. 2012, 45, 455–481. [Google Scholar] [CrossRef]
- Lang, C.X.; Zhang, Y.M.; Mao, Y.W.; Yang, X.Y.; Wang, X.Y.; Luo, X.; Dong, P.C.; Zhu, L.X. Acid tolerance response of Salmonella during simulated chilled beef storage and its regulatory mechanism based on the PhoP/Q system. Food Microbiol. 2021, 95, 103716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Kadim, M.I.; Khoo, W.J.; Zheng, Q.W.; Setyawati, M.I.; Shin, Y.J.; Lee, S.C.; Yuk, H.G. Membrane lipid composition and stress/virulence related gene expression of Salmonella Enteritidis cells adapted to lactic acid and trisodium phosphate and their resistance to lethal heat and acid stress. Int. J. Food Microbiol. 2014, 191, 24–31. [Google Scholar] [CrossRef]
- Modrzyński, J.J.; Christensen, J.H.; Brandt, K.K. Evaluation of dimethyl sulfoxide (DMSO) as a co-solvent for toxicity testing of hydrophobic organic compounds. Ecotoxicology 2019, 28, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed. CLSI: Wayne, PA, USA, 2023.
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, H.H.; Dong, Y.; Wang, G.Y.; Xu, X.L.; Zhou, G.H. Effect of growth media on gene expression levels in Salmonella Typhimurium biofilm formed on stainless steel surface. Food Control 2016, 59, 546–552. [Google Scholar] [CrossRef]
- Carvalho, F.; Atilano, M.L.; Pombinho, R.; Covas, G.; Gallo, R.L.; Filipe, S.R.; Sousa, S.; Cabanes, D. L-Rhamnosylation of Listeria monocytogenes Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane. PLoS Pathog. 2015, 11, e1004919. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.E.V.; Stamford, T.L.M.; Neto, N.J.G.; de Souza, E.L. Inhibition of Staphylococcus aureus in broth and meat broth using synergies of phenolics and organic acids. Int. J. Food Microbiol. 2010, 137, 312–316. [Google Scholar] [CrossRef]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods 2021, 10, 2293. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, H.; Lin, Z.; Xu, P. Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol. Adv. 2015, 33, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Ikeda, J.; Sofos, J. Evaluation of the pH-dependent, stationary-phase acid tolerance in Listeria monocytogenes and Salmonella Typhimurium DT104 induced by culturing in media with 1% glucose: A comparative study with Escherichia coli O157: H7. J. Appl. Microbiol. 2003, 95, 563–575. [Google Scholar] [CrossRef]
- Hu, S.F.; Yu, Y.G.; Lv, Z.Q.; Shen, J.Z.; Ke, Y.B.; Xiao, X.L. Proteomics study unveils ROS balance in acid-adapted Salmonella enteritidis. Food Microbiol. 2020, 92, 103585. [Google Scholar] [CrossRef]
- Alvarez-Ordonez, A.; Fernandez, A.; Bernardo, A.; Lopez, M. Acid tolerance in Salmonella Typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol 2010, 27, 44–49. [Google Scholar] [CrossRef]
- Greenacre, E.; Brocklehurst, T. The acetic acid tolerance response induces cross-protection to salt stress in Salmonella Typhimurium. Int. J. Food Microbiol. 2006, 112, 62–65. [Google Scholar] [CrossRef]
- Lianou, A.; Nychas, G.-J.E.; Koutsoumanis, K.P. Strain variability in biofilm formation: A food safety and quality perspective. Food Res. Int. 2020, 137, 109424. [Google Scholar] [CrossRef]
- Lytou, A.E.; Tzortzinis, K.; Skandamis, P.N.; Nychas, G.-J.E.; Panagou, E.Z. Investigating the influence of organic acid marinades, storage temperature and time on the survival/inactivation interface of Salmonella on chicken breast fillets. Int. J. Food Microbiol. 2019, 299, 47–57. [Google Scholar] [CrossRef]
- da Cunha, N.B.; Cobacho, N.B.; Viana, J.F.C.; Lima, L.A.; Sampaio, K.B.O.; Dohms, S.S.M.; Ferreira, A.C.R.; de la Fuente-Núñez, C.; Costa, F.F.; Franco, O.L.; et al. The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov. Today 2017, 22, 234–248. [Google Scholar] [CrossRef]
- Chaudhari, R.; Singh, K.; Kodgire, P. Biochemical and molecular mechanisms of antibiotic resistance in Salmonella spp. Res. Microbiol. 2023, 174, 103985. [Google Scholar] [CrossRef]
- Lu, H.-F.; Wu, B.-K.; Huang, Y.-W.; Lee, M.-Z.; Li, M.-F.; Ho, H.-J.; Yang, H.-C.; Yang, T.-C. PhoPQ two-component regulatory system plays a global regulatory role in antibiotic susceptibility, physiology, stress adaptation, and virulence in Stenotrophomonas maltophilia. BMC Microbiol. 2020, 20, 312. [Google Scholar] [CrossRef]
- Arunima, A.; Swain, S.K.; Ray, S.; Prusty, B.K.; Suar, M. RpoS-regulated SEN1538 gene promotes resistance to stress and influences Salmonella enterica serovar enteritidis virulence. Virulence 2020, 11, 295–314. [Google Scholar] [CrossRef]
- Burin, R.C.K.; Silva, A.; Nero, L.A. Influence of lactic acid and acetic acid on Salmonella spp. growth and expression of acid tolerance-related genes. Food Res. Int. 2014, 64, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K. Complexity of lipopolysaccharide modifications in Salmonella enterica: Its effects on endotoxin activity, membrane permeability, and resistance to antimicrobial peptides. Food Res. Int. 2012, 45, 493–501. [Google Scholar] [CrossRef]
- Miller, S.I.; Kukral, A.M.; Mekalanos, J.J. A two-component regulatory system (phoP phoQ) controls Salmonella Typhimurium virulence. Proc. Natl. Acad. Sci. USA 1989, 86, 5054–5058. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.Z.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, L.; Jiao, X.; Lu, F.; Yan, C.; Zeng, X.; Wang, J.; Shi, Y. Mechanism of substrate recognition and transport by an amino acid antiporter. Nature 2010, 463, 828–832. [Google Scholar] [CrossRef]
- Kumariya, R.; Sood, S.K.; Rajput, Y.S.; Saini, N.; Garsa, A.K. Increased membrane surface positive charge and altered membrane fluidity leads to cationic antimicrobial peptide resistance in Enterococcus faecalis. Biochim. Et Biophys. Acta (BBA)–Biomembr. 2015, 1848, 1367–1375. [Google Scholar] [CrossRef]
- Petrou, V.I.; Herrera, C.M.; Schultz, K.M.; Clarke, O.B.; Vendome, J.; Tomasek, D.; Banerjee, S.; Rajashankar, K.R.; Belcher Dufrisne, M.; Kloss, B.; et al. Structures of aminoarabinose transferase ArnT suggest a molecular basis for lipid A glycosylation. Science 2016, 351, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Touzé, T.; Tran, A.X.; Hankins, J.V.; Mengin-Lecreulx, D.; Trent, M.S. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol. Microbiol. 2008, 67, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Ling, Z.R.; Dong, Y.J.; Qiao, L.; Shen, Y.B.; Liu, Z.H.; Wu, Y.F.; Li, W.; Zhang, R.; Walsh, T.R.; et al. Mobile Colistin Resistance Enzyme MCR-3 Facilitates Bacterial Evasion of Host Phagocytosis. Adv. Sci. 2021, 8, 2101336. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Song, J.; Velkov, T.; Wang, L.; Zhu, Y.; Li, J. Regulating polymyxin resistance in Gram-negative bacteria: Roles of two-component systems PhoPQ and PmrAB. Future Microbiol. 2020, 15, 445–459. [Google Scholar] [CrossRef]
- Samantha, A.; Vrielink, A. Lipid A Phosphoethanolamine Transferase: Regulation, Structure and Immune Response. J. Mol. Biol. 2020, 432, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Plesa, M.; Hernalsteens, J.P.; Vandenbussche, G.; Ruysschaert, J.M.; Cornelis, P. The SlyB outer membrane lipoprotein of Burkholderia multivorans contributes to membrane integrity. Res. Microbiol. 2006, 157, 582–592. [Google Scholar] [CrossRef]
- Bonnington, K.; Kuehn, M. Breaking the bilayer: OMV formation during environmental transitions. Microb. Cell 2017, 4, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, H.; Lee, S.; Kim, S.; Choi, K.H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015, 72, 25–36. [Google Scholar] [CrossRef]
- Pérez, A.; Canle, D.; Latasa, C.; Poza, M.; Beceiro, A.; Tomás, M.d.M.; Fernández, A.; Mallo, S.; Pérez, S.; Molina, F.; et al. Cloning, Nucleotide Sequencing, and Analysis of the AcrAB-TolC Efflux Pump of Enterobacter cloacae and Determination of Its Involvement in Antibiotic Resistance in a Clinical Isolate. Antimicrob. Agents Chemother. 2007, 51, 3247–3253. [Google Scholar] [CrossRef]
- Muller, R.T.; Pos, K.M. The assembly and disassembly of the AcrAB-TolC three-component multidrug efflux pump. Biol. Chem. 2015, 396, 1083–1089. [Google Scholar] [CrossRef]
- Olubisose, E.T.; Ajayi, A.; Adeleye, A.I.; Smith, S.I. Molecular and phenotypic characterization of efflux pump and biofilm in multi-drug resistant non-typhoidal Salmonella Serovars isolated from food animals and handlers in Lagos Nigeria. One Health Outlook 2021, 3, 2. [Google Scholar] [CrossRef]
- Krojer, T.; Sawa, J.; Schäfer, E.; Saibil, H.R.; Ehrmann, M.; Clausen, T. Structural basis for the regulated protease and chaperone function of DegP. Nature 2008, 453, 885–890. [Google Scholar] [CrossRef]
- Ulvatne, H.; Haukland, H.H.; Samuelsen, Ø.; Krämer, M.; Vorland, L.H. Proteases in Escherichia coli and Staphylococcus aureus confer reduced susceptibility to lactoferricin B. J. Antimicrob. Chemother. 2002, 50, 461–467. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′-3′) | Function | References |
|---|---|---|---|
| 16S rRNA | F: CAGCCACACTGGAACTGAGA | 16 S ribosomal RNA | [31] |
| R: GTGCTTCTTCTGCGGGTAAC | |||
| arnA | F: ACCAGAACCCAGTTTAGCGG | Bifunctional UDP-4-amino-4-deoxy-L-arabinose formyltransferase | This study |
| R: CCGCAACCTGTTAAGCGAAG | |||
| arnT | F: TCGGTGCGAAACAGGAAAGA | Lipid IV(A) 4-amino-4-deoxy-L-arabinosyltransferase | |
| R: GCGAAACGGCGCATTCTATT | |||
| eptA | F: GGCGAGTGCTACGATGAAGT | Phosphoethanolamine transferase EptA | |
| R: TGTAATAGGTTGGGCCGTGG | |||
| pagP | F: TTCAGTCTCTGCGGCGGATAA | Lipid IV(A) palmitoyltransferase PagP | |
| R:GGTAATGGCGGGGACATACAAATC | |||
| slyB | F: TTATCCCTAGCGGGGTGTGT | Outer membrane lipoprotein SlyB | |
| R:CCCTGAATTTGAACCGGACG | |||
| pgtE | F: CGGACACCAGCGTCAACTAT | Omptin family outer membrane protease PgtE | |
| R: CGCCTTGTAGTTATCGCCCT | |||
| degP | F: TTCACCTGGCCGTATTCCAC | Serine endoprotease DegP | |
| R: CTGGTGAACCTGAACGGTGA | |||
| baeR | F: TCATTAACGGGCTTTCGGCA | Response regulator in two-component regulatory system with BaeS | |
| R: AATCGAAGAGATCGACCGGC | |||
| tolC | F: GATACAGCGGCAGGGAGAAG | TolC family outer membrane protein | |
| R: CCAACTCCACCCAGTACGAC |
| Antibiotics | Acid Adaptation Treatment | WT | ∆phoP |
|---|---|---|---|
| Polymyxin B | Non-adapted | 4(I) | 2(I) |
| Acid-adapted | 32(R) | 2(I) | |
| Ceftazidime | Non-adapted | 0.5(S) | 0.25(S) |
| Acid-adapted | 1(S) | 1(S) | |
| Gentamycin | Non-adapted | 8(R) | 8(R) |
| Acid-adapted | 16(R) | 16(R) | |
| Ampicillin | Non-adapted | 2(S) | 1(S) |
| Acid-adapted | 2(S) | 1(S) |
| Strain | pH | |
|---|---|---|
| 7 | 5.4 | |
| WT | 75.95 ± 3.66 bx | 81.51 ± 2.65 ax |
| ∆phoP | 70.15 ± 2.92 by | 72.04 ± 2.98 ay |
| Storage Time (d) | Medium | Strain and pH | |||
|---|---|---|---|---|---|
| WT | ∆phoP | ||||
| 7 | 5.4 | 7 | 5.4 | ||
| 1 | Neutral ME | 4(R) | 4(R) | 2(I) | 2(I) |
| Acidic ME | 16(R) | 32(R) | 1(I) | 2(I) | |
| 7 | Neutral ME | 2(I) | 2(I) | 1(I) | 1(I) |
| Acidic ME | 16(R) | 32(R) | 1(I) | 1(I) | |
| 14 | Neutral ME | 1(I) | 2(I) | 2(I) | 2(I) |
| Acidic ME | 16(R) | 32(R) | 1(I) | 0.5(I) | |
| 21 | Neutral ME | 2(I) | 2(I) | 2(I) | 2(I) |
| Acidic ME | 16(R) | 16(R) | 1(I) | 1(I) | |
| 28 | Neutral ME | 1(I) | 1(I) | 0.5(I) | 0.5(I) |
| Acidic ME | 4(R) | 8(R) | 0.5(I) | 0.5(I) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Han, J.; Zhu, L.; Nychas, G.-J.E.; Mao, Y.; Yang, X.; Liu, Y.; Jiang, X.; Zhang, Y.; Dong, P. The Effect of the PhoP/PhoQ System on the Regulation of Multi-Stress Adaptation Induced by Acid Stress in Salmonella Typhimurium. Foods 2024, 13, 1533. https://doi.org/10.3390/foods13101533
Gao X, Han J, Zhu L, Nychas G-JE, Mao Y, Yang X, Liu Y, Jiang X, Zhang Y, Dong P. The Effect of the PhoP/PhoQ System on the Regulation of Multi-Stress Adaptation Induced by Acid Stress in Salmonella Typhimurium. Foods. 2024; 13(10):1533. https://doi.org/10.3390/foods13101533
Chicago/Turabian StyleGao, Xu, Jina Han, Lixian Zhu, George-John E. Nychas, Yanwei Mao, Xiaoyin Yang, Yunge Liu, Xueqing Jiang, Yimin Zhang, and Pengcheng Dong. 2024. "The Effect of the PhoP/PhoQ System on the Regulation of Multi-Stress Adaptation Induced by Acid Stress in Salmonella Typhimurium" Foods 13, no. 10: 1533. https://doi.org/10.3390/foods13101533






