Regenerative Efficacy of Supercritical Carbon Dioxide-Derived Bone Graft Putty in Rabbit Bone Defect Model
Abstract
1. Introduction
2. Materials and Methods
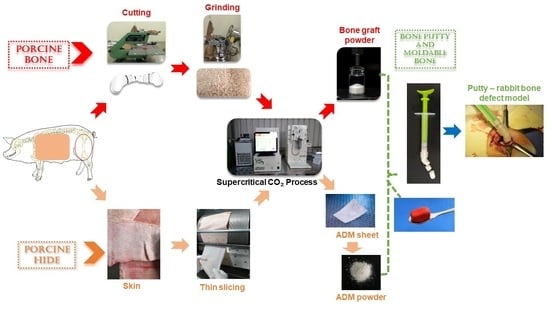
2.1. Development of Bone Graft
2.2. Development of Acellular Dermal Matrix Powder
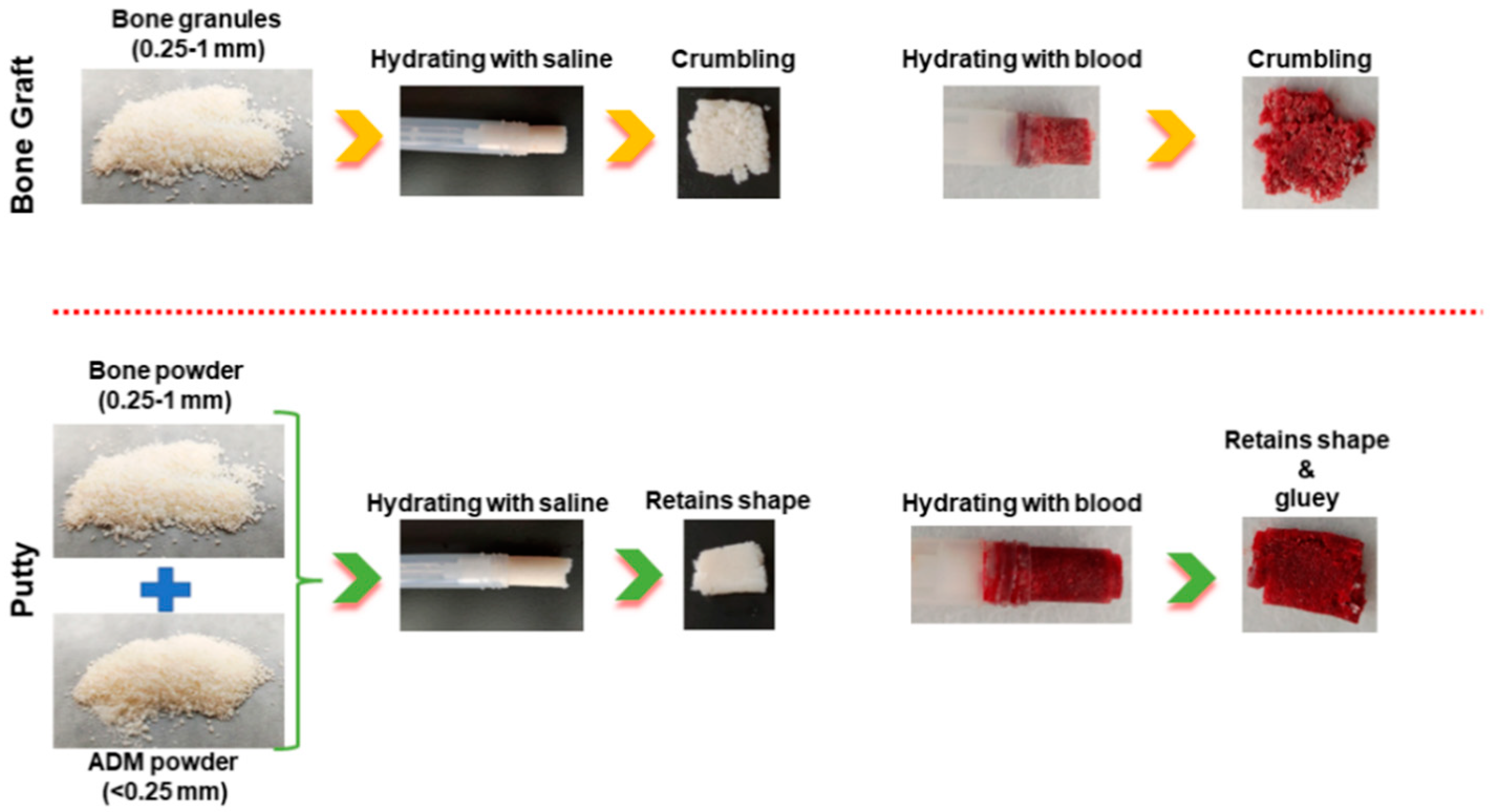
2.3. Development of Putty from SCCO2-Derived Bone Graft Powder and Acellular Dermal Matrix Powder
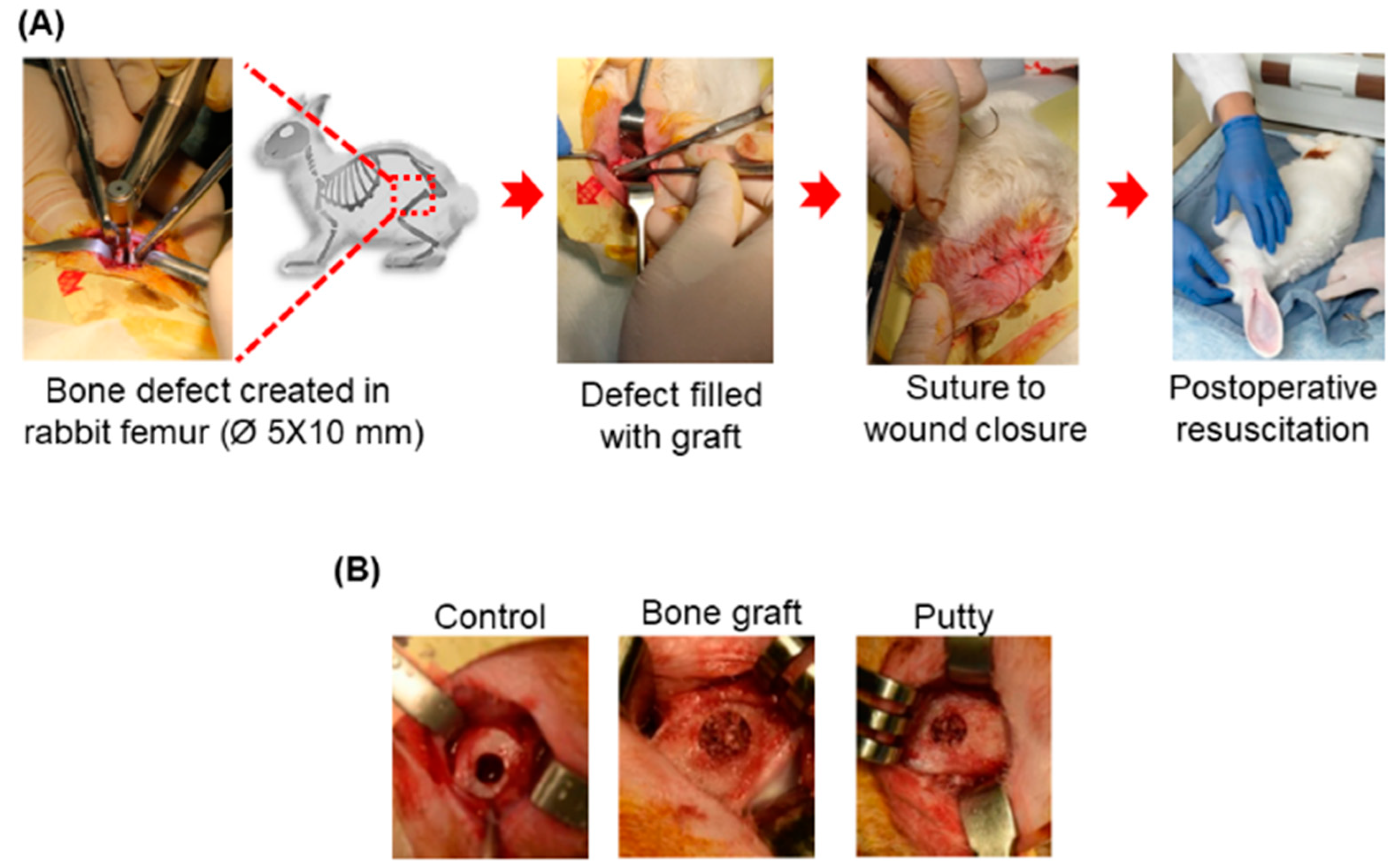
2.4. Animal Efficacy Test—Rabbit Femoral Bone Defect Model
2.4.1. Animals
2.4.2. Surgical Procedure
2.4.3. Observations and Euthanasia
2.4.4. X-ray and Micro-CT Analysis
2.4.5. Histological Analysis
2.4.6. Hematoxylin and Eosin Staining
2.4.7. Masson’s Trichrome Staining
2.4.8. Alizarin Red S Staining
2.5. Statistical Methods
3. Results
3.1. Physical Comparison of Bone Graft and Putty
3.2. Clinical Investigation: General Gross Observations
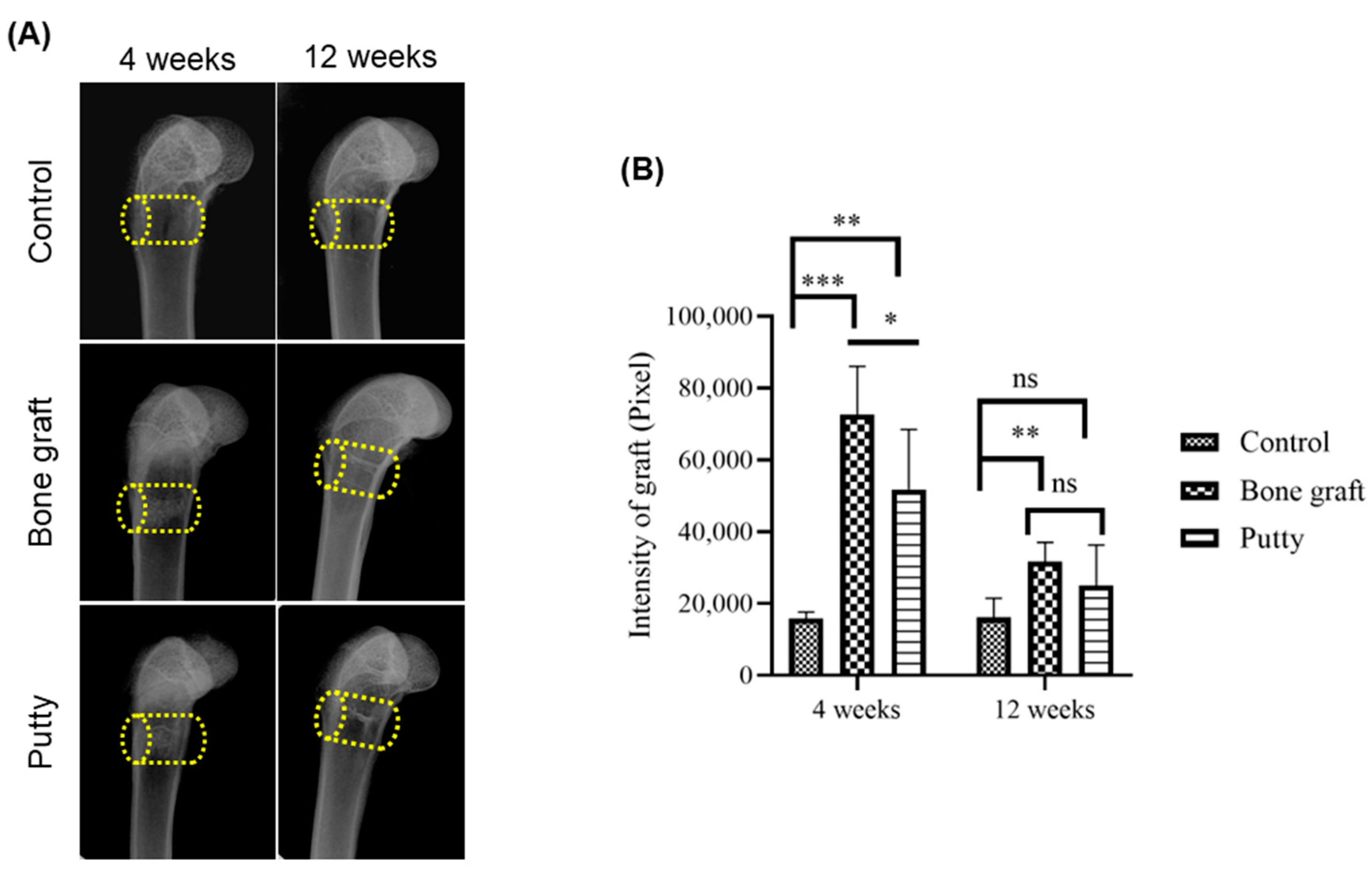
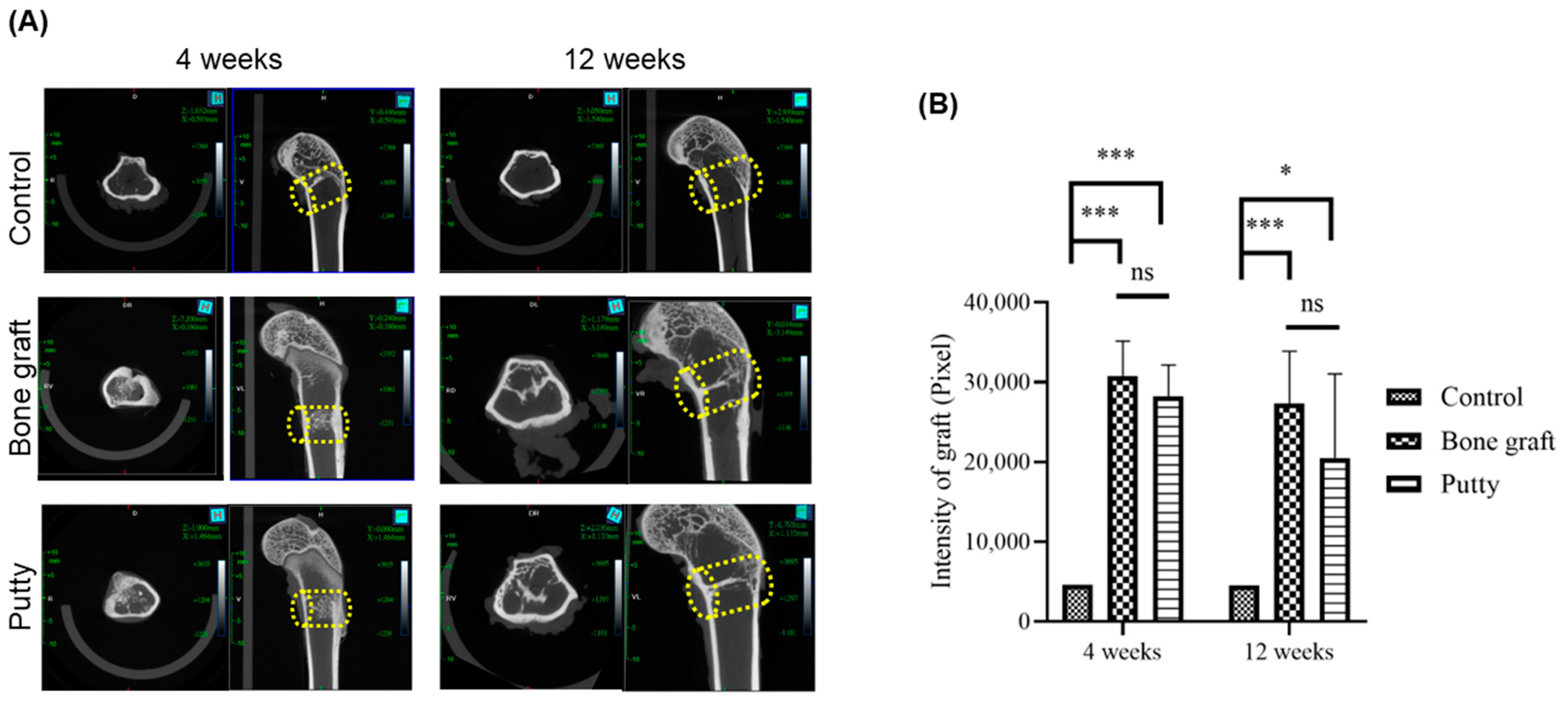
3.3. Radiographic Evaluation of Bone Graft and Putty-Implanted Animals
3.4. Role of Putty on Osteogenesis by Micro-CT Examination
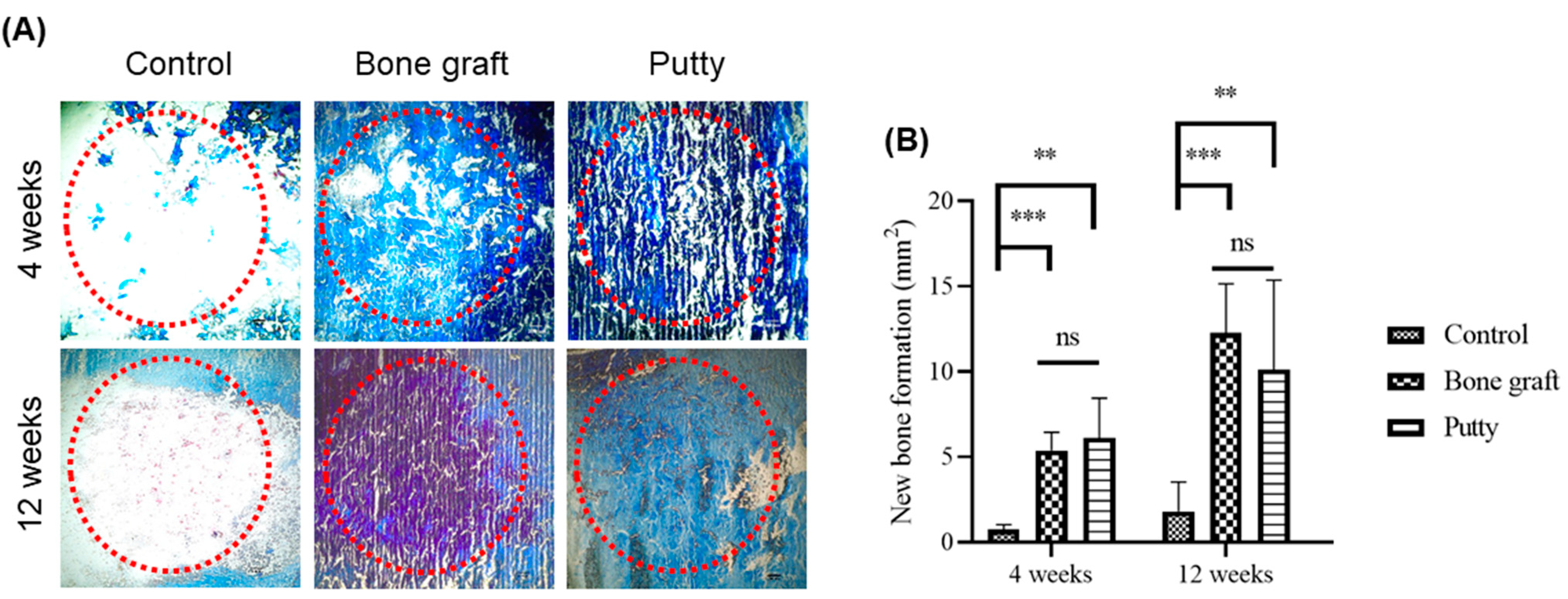
3.5. Role of Putty on Osteogenesis by Histological Assessment
3.6. Role of Putty on Osteogenesis by Masson Trichrome Staining in the Bone Defect Area
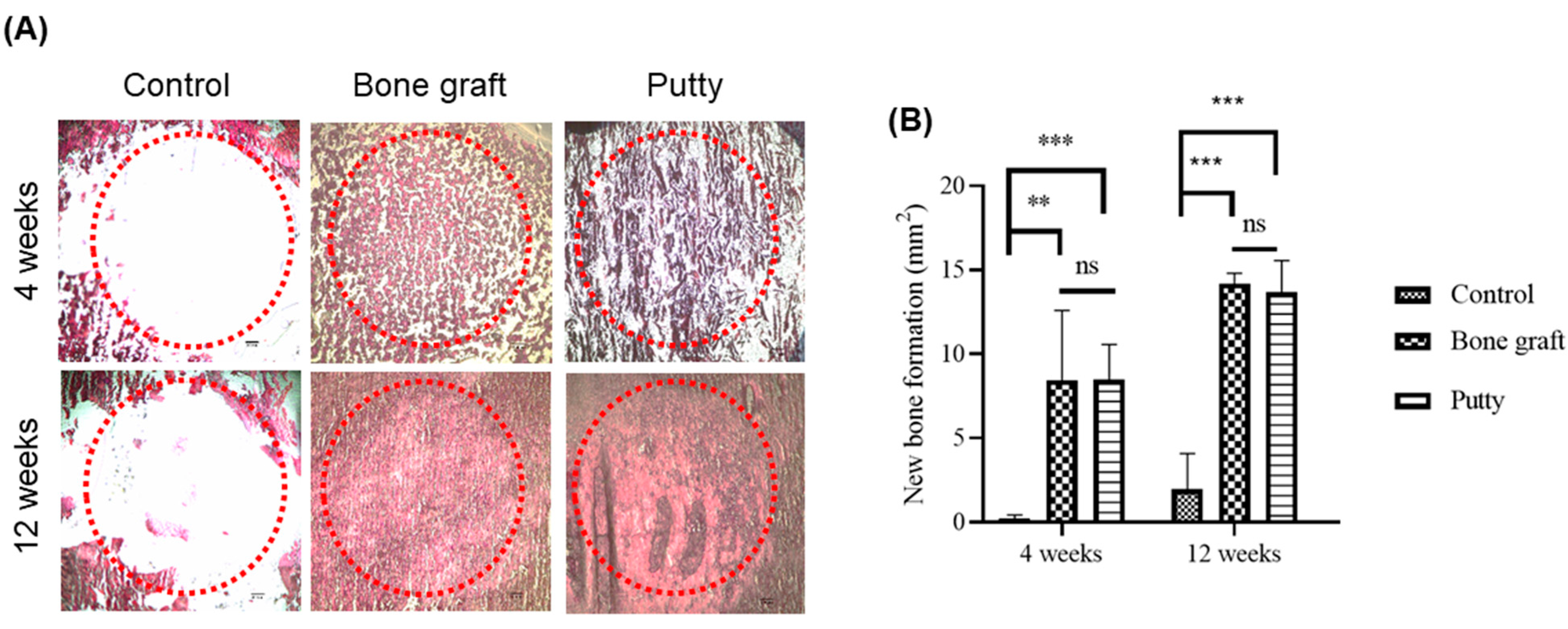
3.7. Role of Putty on Osteogenesis by Alizarin Red S Staining in the Bone Defect Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schlickewei, W.; Schlickewei, C. The Use of Bone Substitutes in the Treatment of Bone Defects—The Clinical View and History. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Chen, I.C.; Su, C.Y.; Lai, C.C.; Tsou, Y.S.; Zheng, Y.; Fang, H.W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. J. Funct. Biomater. 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Bi, Q.; Zhao, C.; Chen, J.-Y.; Cai, M.-H.; Chen, X.-Y. Recent Advances in Biomaterials for the Treatment of Bone Defects. Organogenesis 2020, 16, 113–125. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar]
- Gardin, C.; Ricci, S.; Ferroni, L.; Guazzo, R.; Sbricoli, L.; De Benedictis, G.; Zavan, B. Decellurisation and delipidation protocols of bovine bone and pericardium for bone grafting and guided bone regeneration procedures. PLoS ONE 2015, 10, e0132344. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hsieh, D.J.; Periasamy, S.; Yen, K.C.; Wang, H.C.; Chien, H.H. Development of a decellularized porcine bone graft by supercritical carbon dioxide extraction technology for bone regeneration. J. Tissue Eng. Regen. Med. 2021, 15, 401–414. [Google Scholar] [CrossRef]
- Chou, P.R.; Lin, Y.N.; Wu, S.H.; Lin, S.D.; Srinivasan, P.; Hsieh, D.J.; Huang, S.H. Supercritical Carbon Dioxide-decellularized Porcine Acellular Dermal Matrix combined with Autologous Adipose-derived Stem Cells: Its Role in Accelerated Diabetic Wound Healing. Int. J. Med. Sci. 2020, 17, 354–367. [Google Scholar] [CrossRef]
- AVMA Panel on Euthanasia. American Veterinary Medical Association. 2000 Report of the AVMA Panel on Euthanasia. J. Am. Vet. Med. Assoc. 2001, 218, 669–696. [Google Scholar] [CrossRef]
- Omata, K.; Matsuno, T.; Asano, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Enhanced bone regeneration by gelatin-β-tricalcium phosphate composites enabling controlled release of bFGF. J. Tissue Eng. Regen. Med. 2014, 8, 604–611. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, T.; Li, A.; Liu, B.; Cui, Z.; Qiao, Y.; Tian, Y.; Qiu, D. Porous Particle-Reinforced Bioactive Gelatin Scaffold for Large Segmental Bone Defect Repairing. ACS Appl. Mater. Interfaces 2018, 10, 6956–6964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yin, F.; Lin, Y.; Xia, W.; Zhou, L.; Pan, C.; Wang, N.; Shan, H.; Zhou, Z.; Yu, X. The promotion of bone regeneration through CS/GP-CTH/antagomir-133a/b sustained release system. Nanomedicine 2020, 24, 102116. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, W.; Zhang, L.; Wang, Y.; Zhai, Z.; Liu, F. The effect of enhanced bone marrow in conjunction with 3D-printed PLA-HA in the repair of critical-sized bone defects in a rabbit model. Ann. Transl. Med. 2021, 9, 1134. [Google Scholar] [CrossRef]
- Walsh, W.R.; Oliver, R.A.; Christou, C.; Lovric, V.; Walsh, E.R.; Prado, G.R.; Haider, T. Critical Size Bone Defect Healing Using Collagen-Calcium Phosphate Bone Graft Materials. PLoS ONE 2017, 12, e0168883. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef]
- Sadek, A.A.; Abd-Elkareem, M.; Abdelhamid, H.N.; Moustafa, S.; Hussein, K. Enhancement of critical-sized bone defect regeneration using UiO-66 nanomaterial in rabbit femurs. BMC Vet. Res. 2022, 8, 260. [Google Scholar] [CrossRef]
- Zhou, T.; Li, G.; Lin, S.; Tian, T.; Ma, Q.; Zhang, Q.; Shi, S.; Xue, C.; Ma, W.; Cai, X.; et al. Electrospun Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/Graphene Oxide Scaffold: Enhanced Properties and Promoted in Vivo Bone Repair in Rats. ACS Appl. Mater. Interfaces 2017, 9, 42589–42600. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Biophys. Acta 2018, 1862, 936–945. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, L.; Yang, X.G.; Wang, F.; Feng, J.T.; Hua, K.C.; Li, Q.; Hu, Y.C. Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Schallenberger, M.A.; Rossmeier, K.; Lovick, H.M.; Meyer, T.R.; Aberman, H.M.; Juda, G.A. Comparison of the osteogenic potential of OsteoSelect demineralized bone matrix putty to NovaBone calcium-phosphosilicate synthetic putty in a cranial defect model. J. Craniofac. Surg. 2014, 25, 657–661. [Google Scholar] [CrossRef]
- Nannmark, U.; Sennerby, L. The Bone Tissue Responses to Prehydrated and Collagenated Cortico-Cancellous Porcine Bone Grafts: A Study in Rabbit Maxillary Defects. Clin. Implant. Dent. Relat. Res. 2008, 10, 264–270. [Google Scholar] [CrossRef]
- Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef]
- Barone, A.; Ricci, M.; Grassi, R.F.; Nannmark, U.; Quaranta, A.; Covani, U.; Grassi, F.R. A 6-month histological analysis on maxillary sinus augmentation with and without use of collagen membranes over the osteotomy window: Randomized clinical trial. Clin. Oral Implant. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef]
- AbdelGawad, M.E.; Søe, K.; Andersen, T.L.; Merrild, D.M.; Christiansen, P.; Kjærsgaard-Andersen, P.; Delaisse, J.-M. Does collagen trigger the recruitment of osteoblasts into vacated bone resorption lacunae during bone remodeling? Bone 2014, 67, 181–188. [Google Scholar] [CrossRef]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; Whitlock, P.W. A decellularized porcine xenograftderived bone scaffold for clinical use as a bone graft substitute: A critical evaluation of processing and structure. J. Funct. Biomater. 2018, 9, 45. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, W.; Yang, L.; Liang, C.; Xu, B. Improved the biocompatibility of cancellous bone with compound physico-chemical decellularization process. Regen. Biomater. 2020, 7, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, M.; Hsieh, D.; Periasamy, S.; Yen, K.; Chuang, C.; Wang, H.; Tseng, F.; Kuo, J.; Chien, H. Evaluating the bone-regenerative role of the decellularized porcine bone xenograft in a canine extraction socket model. Clin. Exp. Dent. Res. 2021, 7, 409–418. [Google Scholar] [CrossRef]
- Farrokhi, A.; Pakyari, M.; Nabai, L.; Pourghadiri, A.; Hartwell, R.; Jalili, R.; Ghahary, A. Evaluation of detergent-free and detergent-based methods for decellularization of murine skin. Tissue Eng. Part A 2018, 24, 955–967. [Google Scholar] [CrossRef]
- Ge, L.; Zheng, S.; Wei, H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burns 2009, 35, 46–50. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, D.J.; Periasamy, S.; Chuang, C.T.; Tseng, F.W.; Kuo, J.C.; Tarng, Y.W. Regenerative porcine dermal collagen matrix developed by supercritical carbon dioxide extraction technology: Role in accelerated wound healing. Materialia 2020, 9, 100576. [Google Scholar] [CrossRef]
- Qiu, J.; Li, J.; Wang, G.; Zheng, L.; Ren, N.; Liu, H.; Tang, W.; Jiang, H.; Wang, Y. In vitro investigation on the biodegradability and biocompatibility of genipin cross-linked porcine acellular dermal matrix with intrinsic fluorescence. ACS Appl. Mater. Interfaces 2013, 5, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Inderdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Kuna, V.K.; Padma, A.M.; Håkansson, J.; Nygren, J.; Sjöback, R.; Petronis, S.; Sumitran-Holgersson, S. Significantly accelerated wound healing of full-thickness skin using a novel composite gel of porcine acellular dermal matrix and human peripheral blood cells. Cell Transplant. 2017, 26, 293–307. [Google Scholar] [CrossRef] [PubMed]


| Groups | Implantation | Number of Animals |
|---|---|---|
| Control | None | 8 |
| Bone graft | Bone graft | 12 |
| Putty | Putty | 12 |
| Properties | Bone Graft | Putty |
|---|---|---|
| Composition | Bone powder | Bone powder and ADM powder |
| Physical form | Powder | Putty |
| Chemical nature | Natural bone minerals and collagen | Natural bone minerals and collagen + natural dermal collagen |
| Pliability | Not applicable | Versatile |
| Usage | Needs surgical tools | Ready to use after rehydration |
| Stability/ retaining nature in fluid | Easily crumbles | Retains the shape and structure |
| Adhesiveness | Low | Relatively good |
| Osseointegration | Good | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-L.; Luo, Y.-L.; Chen, Y.-W.; Wu, C.-T.; Periasamy, S.; Yen, K.-C.; Hsieh, D.-J. Regenerative Efficacy of Supercritical Carbon Dioxide-Derived Bone Graft Putty in Rabbit Bone Defect Model. Biomedicines 2022, 10, 2802. https://doi.org/10.3390/biomedicines10112802
Chiu Y-L, Luo Y-L, Chen Y-W, Wu C-T, Periasamy S, Yen K-C, Hsieh D-J. Regenerative Efficacy of Supercritical Carbon Dioxide-Derived Bone Graft Putty in Rabbit Bone Defect Model. Biomedicines. 2022; 10(11):2802. https://doi.org/10.3390/biomedicines10112802
Chicago/Turabian StyleChiu, Yen-Lung, Yun-Li Luo, Yuan-Wu Chen, Chi-Tsung Wu, Srinivasan Periasamy, Ko-Chung Yen, and Dar-Jen Hsieh. 2022. "Regenerative Efficacy of Supercritical Carbon Dioxide-Derived Bone Graft Putty in Rabbit Bone Defect Model" Biomedicines 10, no. 11: 2802. https://doi.org/10.3390/biomedicines10112802
APA StyleChiu, Y.-L., Luo, Y.-L., Chen, Y.-W., Wu, C.-T., Periasamy, S., Yen, K.-C., & Hsieh, D.-J. (2022). Regenerative Efficacy of Supercritical Carbon Dioxide-Derived Bone Graft Putty in Rabbit Bone Defect Model. Biomedicines, 10(11), 2802. https://doi.org/10.3390/biomedicines10112802