Association between Height-Changing Scores and Risk of Sarcopenia Estimated from Anthropometric Measurements in Older Adults: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
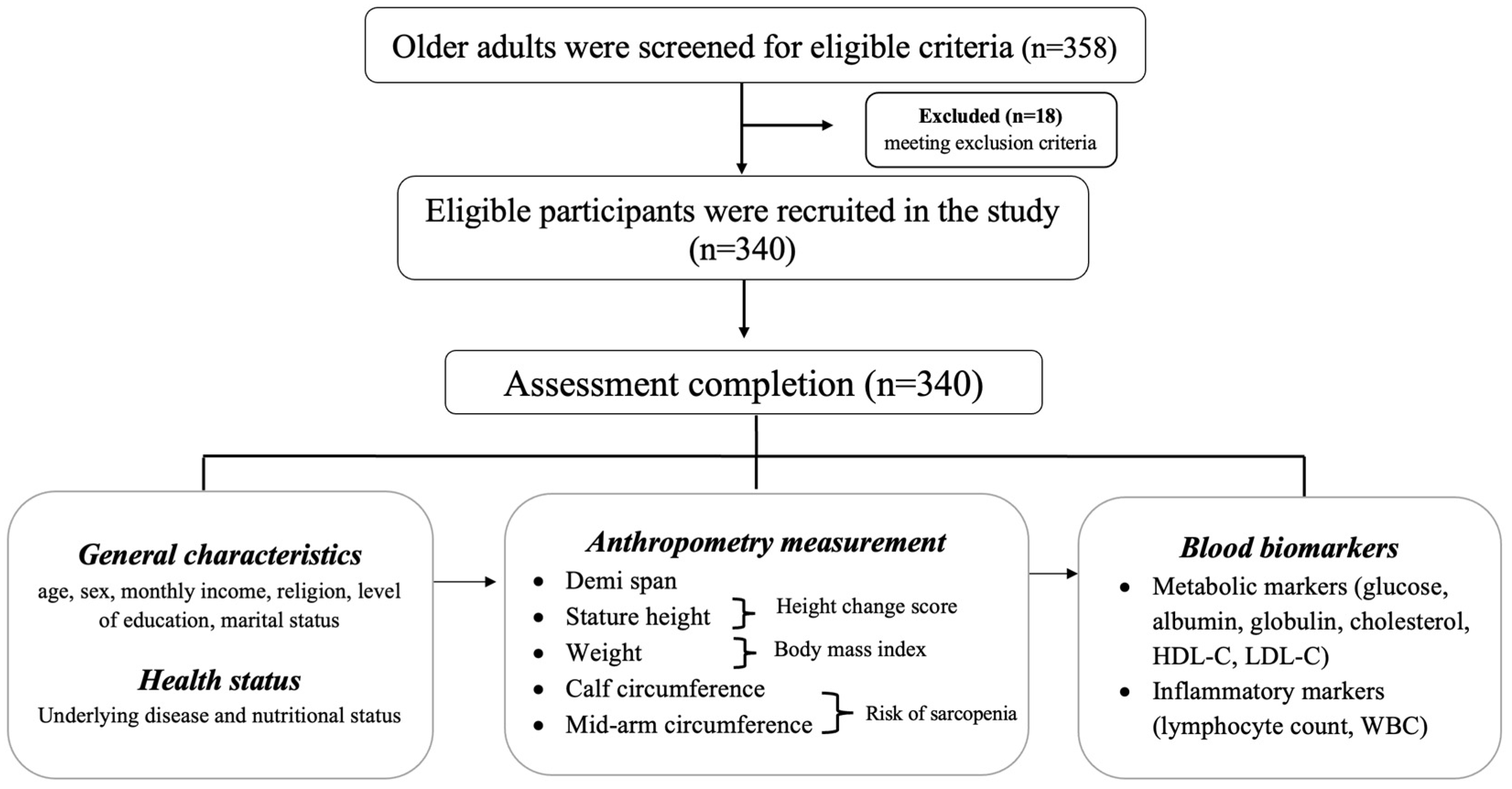
2.1. Participants
2.2. Data Collection
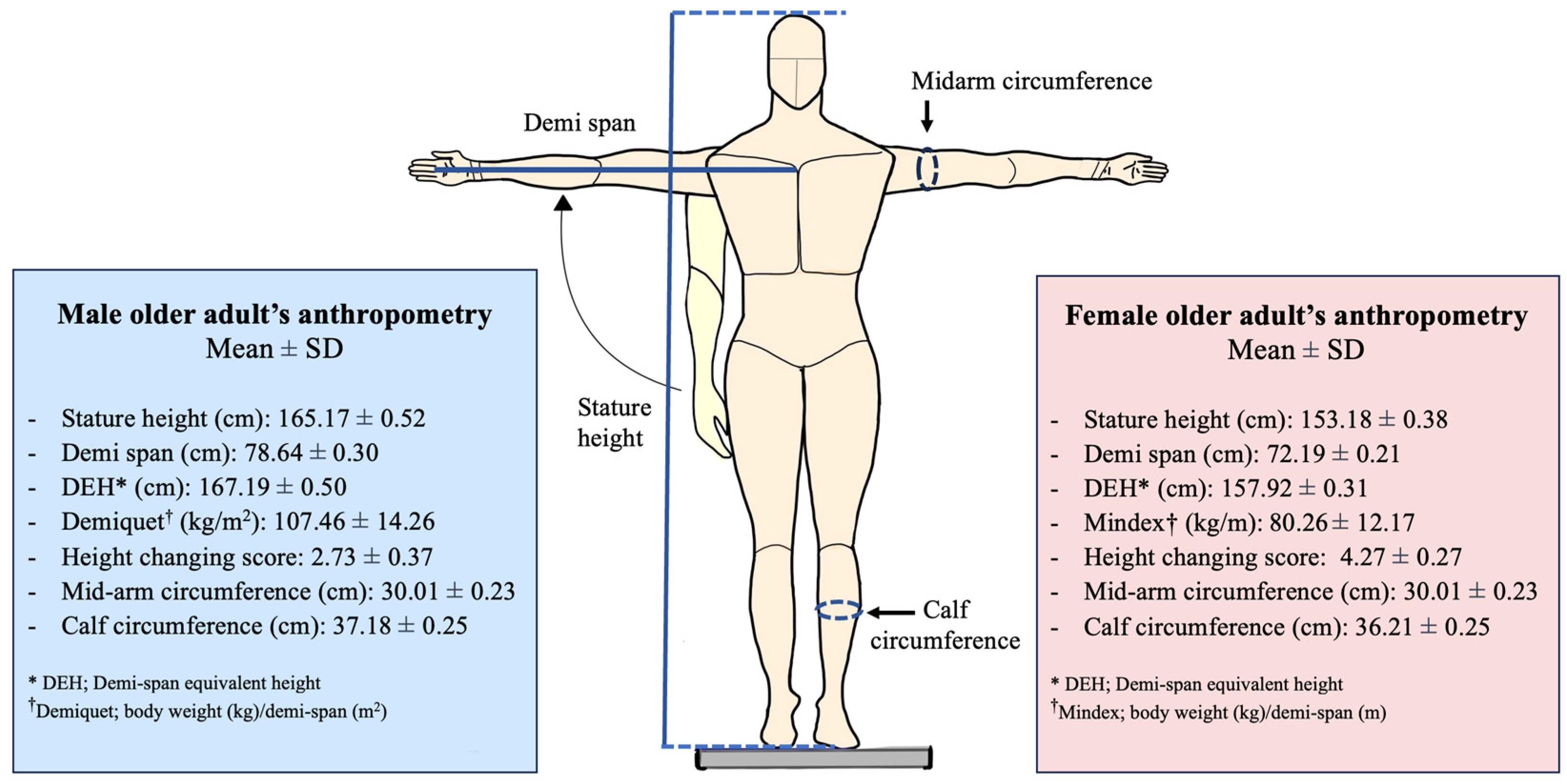
2.3. Measurement
- Calf circumference less than 34 cm for men and 33 cm for women [20].
- A mid-arm circumference of ≤28.6 cm for men and ≤27.5 cm for women [7].
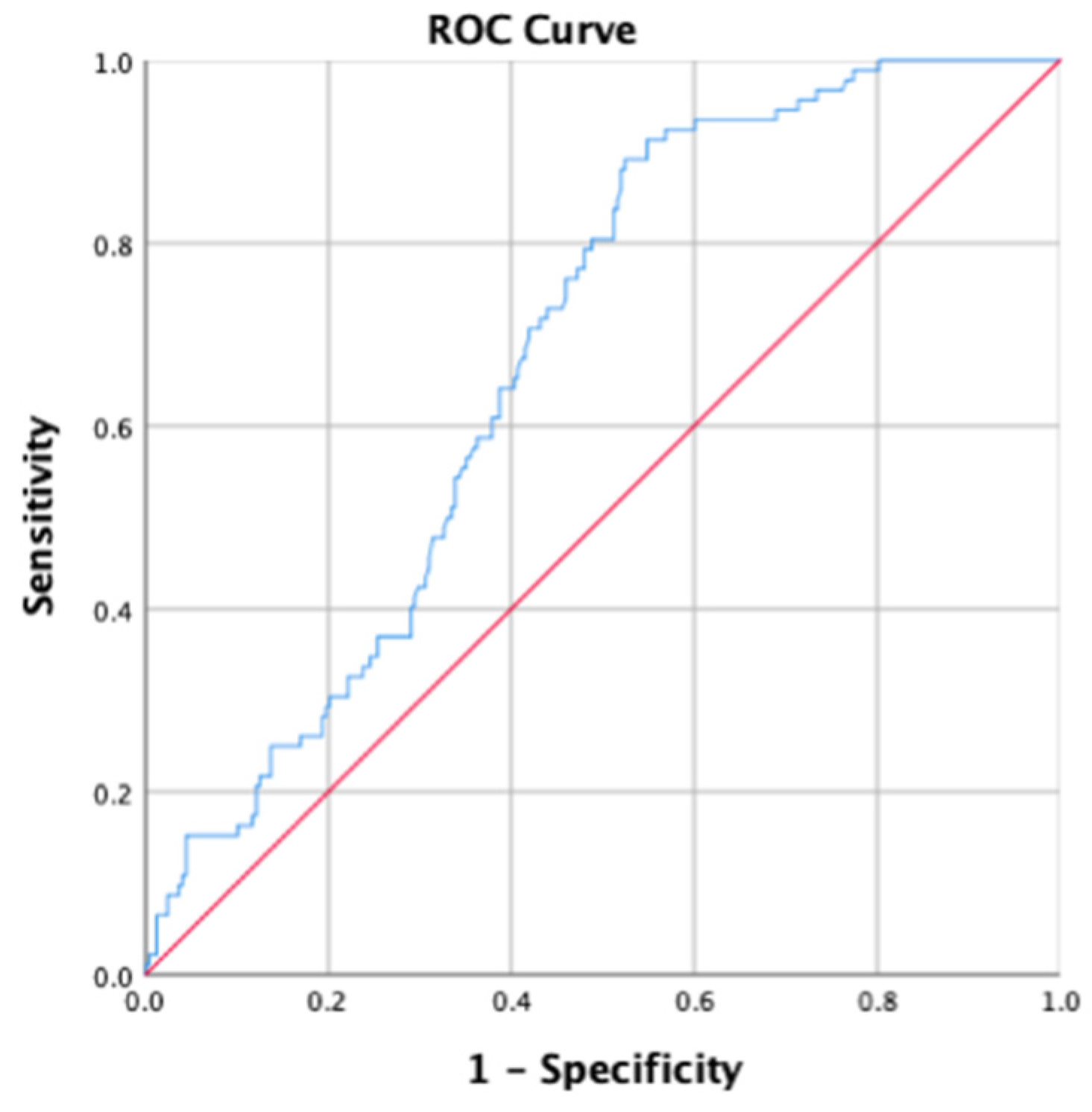
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Association between HCS and Anthropometric Measurement
3.3. Blood Biomarkers and Risk of Sarcopenia Defined by Anthropometry Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ageing (World Health Organization). Available online: https://www.who.int/health-topics/ageing#tab=tab_1 (accessed on 22 December 2023).
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Koyanagi, A.; Cereda, E.; Maggi, S.; Barbagallo, M.; Dominguez, L.J.; Smith, L. Sarcopenia reduces quality of life in the long-term: Longitudinal analyses from the English longitudinal study of ageing. Eur. Geriatr. Med. 2022, 13, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.B.; Hannan, M.T.; Murabito, J.M.; Kiel, D.P.; McLean, R.R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Takeuchi, T.; Goto, M.; Ogura, T.; Nakamura, S.; Kakimoto, K.; Miyazaki, T.; Nishiguchi, S.; et al. Screening Tools for Sarcopenia. In Vivo 2021, 35, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.J.; Liu, H.; Liu, X.L.; Jia, S.L.; Hou, L.S.; Xia, X.; Dong, B.R. Mid-Upper Arm Circumference as an Alternative Screening Instrument to Appendicular Skeletal Muscle Mass Index for Diagnosing Sarcopenia. Clin. Interv. Aging 2021, 16, 1095–1104. [Google Scholar] [CrossRef]
- Kawakami, R.; Miyachi, M.; Sawada, S.S.; Torii, S.; Midorikawa, T.; Tanisawa, K.; Ito, T.; Usui, C.; Ishii, K.; Suzuki, K.; et al. Cut-offs for calf circumference as a screening tool for low muscle mass: WASEDA’S Health Study. Ger. Gerontol. Int. 2020, 20, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Ukegbu, P.O.; Kruger, H.S.; Meyer, J.D.; Nienaber-Rousseau, C.; Botha-Ravyse, C.; Moss, S.J.; Kruger, M.I. The association between calf circumference and appendicular skeletal muscle mass index of black urban women in Tlokwe City. J. Endocrinol. Metab. Diabetes S. Afr. 2018, 23, 86–90. [Google Scholar] [CrossRef]
- Aggarwal, S.; Nityanand. Calcium and vitamin D in post menopausal women. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. 3), S618–S620. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Zhang, S.; Hao, Q.; Zhu, B.; Teng, Y.; Long, Q.; Li, S.; Lv, Y.; Yue, Q.; et al. A pooled analysis of the association between sarcopenia and osteoporosis. Medicine 2022, 101, e31692. [Google Scholar] [CrossRef]
- He, C.; He, W.; Hou, J.; Chen, K.; Huang, M.; Yang, M.; Luo, X.; Li, C. Bone and Muscle Crosstalk in Aging. Front. Cell Dev. Biol. 2020, 8, 585644. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Yuguchi, S.; Kamo, T.; Azami, M.; Ogihara, H.; Asano, S. Association of height loss with falls and sarcopenia in community-dwelling older women. Osteoporos. Sarcopenia 2020, 6, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.S.; Fromm, E.; Klenk, J.; Concin, H.; Nagel, G. Change in Height, Weight, and body mass index: Longitudinal data from Austria. Am. J. Hum. Biol. 2014, 26, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, J.D.; Muller, D.C.; Andres, R. Longitudinal Change in Height of Men and Women: Implications for Interpretation of the Body Mass Index: The Baltimore Longitudinal Study of Aging. Am. J. Epidemiol. 1999, 150, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Fernihough, A.; McGovern, M.E. Physical stature decline and the health status of the elderly population in England. Econ. Hum. Biol. 2015, 16, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Mindell, J. A comparison of measured height and demi-span equivalent height in the assessment of body mass index among people aged 65 years and over in England. Age Ageing 2008, 37, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hickson, M.; Frost, G. A comparison of three methods for estimating height in the acutely ill elderly population. J. Hum. Nutr. Diet. 2003, 16, 13–20. [Google Scholar] [CrossRef]
- Bassey, E.J. Demi-span as a measure of skeletal size. Ann. Hum. Biol. 1986, 13, 499–502. [Google Scholar] [CrossRef]
- Rose Berlin Piodena-Aportadera, M.; Lau, S.; Chew, J.; Lim, J.P.; Ismail, N.H.; Ding, Y.Y.; Lim, W.S. Calf Circumference Measurement Protocols for Sarcopenia Screening: Differences in Agreement, Convergent Validity and Diagnostic Performance. Ann. Geriatr. Med. Res. 2022, 26, 215–224. [Google Scholar] [CrossRef]
- De Hond, A.A.H.; Steyerberg, E.W.; van Calster, B. Interpreting area under the receiver operating characteristic curve. Lancet Digit. Health 2022, 4, e853–e855. [Google Scholar] [CrossRef]
- Launer, L.; Harris, T.; on behalf of the Ad Hoc Committee on the Statistics of Anthropometry and Aging. Weight, Height and Body Mass Index Distributions in Geographically and Ethnically Diverse Samples of Older Persons. Age Ageing 1996, 25, 300–306. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Chang, H.-Y.; Mau, L.-W.; Lee, T.-K.; Liu, H.-W. Height, Weight, and Body Mass Index of Elderly Persons in Taiwan. J. Gerontol. Ser. A 2000, 55, M684–M690. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.E.; Paris, M.T.; Avrutin, E.; Mourtzakis, M. Altered features of body composition in older adults with type 2 diabetes and prediabetes compared with matched controls. J. Cachexia Sarcopenia Muscle 2022, 13, 1087–1099. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef]
- Ji, S.; Lee, E.; Kim, B.-J.; Baek, J.Y.; Yi, Y.; Jang, I.-Y.; Jung, H.-W. Height loss as an indicator of ageing through its association with frailty and sarcopenia: An observational cohort study. Arch. Gerontol. Geriatr. 2023, 110, 104916. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Abe, T.; Akai, K.; Kijima, T.; Takeda, M.; Yamasaki, M.; Isomura, M.; Nabika, T.; Yano, S. Height loss but not body composition is related to low back pain in community-dwelling elderlies: Shimane CoHRE study. BMC Musculoskelet. Disord. 2019, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Schousboe, J.T.; Kats, A.M.; Vo, T.N.; Taylor, B.C.; Cawthon, P.M.; Cauley, J.A.; Lane, N.E.; Hoffman, A.R.; Langsetmo, L. Height Loss in Old Age and Fracture Risk Among Men in Late Life: A Prospective Cohort Study. J. Bone Min. Res. 2021, 36, 1069–1076. [Google Scholar] [CrossRef]
- Ahn, K.-S.; Kang, C.H.; Cho, S.B.; Cho, K.-H.; Han, K.-D.; Park, Y.-G.; Kim, Y.-H. Height Loss Was Associated With Osteoporosis in Korean Elderly Men, Not in Women: The Korea National Health and Nutrition Examination Survey 2008–2010. J. Clin. Densitom. 2019, 22, 59–66. [Google Scholar] [CrossRef]
- Siminoski, K.; Jiang, G.; Adachi, J.D.; Hanley, D.A.; Cline, G.; Ioannidis, G.; Hodsman, A.; Josse, R.G.; Kendler, D.; Olszynski, W.P.; et al. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos. Int. 2005, 16, 403–410. [Google Scholar] [CrossRef]
- Mai, X.; Marshall, B.; Hovey, K.M.; Sperrazza, J.; Wactawski-Wende, J. Risk factors for 5-year prospective height loss among postmenopausal women. Menopause 2018, 25, 883–889. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Perissinotto, E.; Pisent, C.; Sergi, G.; Grigoletto, F. Anthropometric measurements in the elderly: Age and gender differences. Br. J. Nutr. 2002, 87, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Nam, G.E.; Han, K.D.; Choi, S.W.; Jung, S.W.; Bok, A.R.; Kim, Y.H.; Lee, K.S.; Han, B.D.; Kim, D.H. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: The 2008-2010 Korea National Health and Nutrition Examination Survey. J. Endocrinol. Investig. 2014, 37, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Z.; Niu, Y.; Li, X.; Zhu, L.; Lu, S.; Zhang, H.; Fan, J.; Ning, G.; Qin, L.; et al. Association of calf circumference with insulin resistance and non-alcohol fatty liver disease: The REACTION study. BMC Endocr. Disord. 2017, 17, 28. [Google Scholar] [CrossRef][Green Version]
- Kim, G.M.; Song, S.; Park, J.H.; Tak, Y.J.; Wang, I.J.; Huh, U.; Cho, J.S. Diagnostic significance of calf circumference in sarcopenia of healthy korean adult males. Front. Physiol. 2022, 13, 973265. [Google Scholar] [CrossRef]

| Factors | Sarcopenia Risk | p-Value | |
|---|---|---|---|
| Risk of Sarcopenia n (%) Overall = 64 | No Risk of Sarcopenia n (%) Overall = 276 | ||
| Gender | |||
| 44 (68.75) | 161 (58.33) | 0.081 f |
| 20 (31.25) | 115 (41.67) | |
| Age (years) Mean (SD) | 69.1 (6.4) | 65.8 (4.8) | 0.001 * |
| Religion | |||
| 57 (89.06) | 260 (94.20) | 0.320 c |
| 7 (10.93) | 16 (5.79) | |
| Marital status | |||
| 49 (76.56) | 204 (73.91) | 0.619 c |
| 15 (23.44) | 72 (26.09) | |
Education level
| 29 (45.31) 5 (7.82) 30 (46.87) | 59 (21.34) 61 (22.1) 156 (56.52) | 0.003 * |
| Income (Thai Baht per month) | |||
| 31 (48.43) | 74 (26.81) | 0.002 * |
| 21 (32.82) | 99 (35.86) | |
| 12 (18.75) | 103 (37.32) | |
| Underlying disease | |||
| 7 (10.94) | 46 (16.57) | 0.198 f |
| 21 (32.81) | 115 (41.67) | 0.163 f |
| 58 (90.63) | 254 (92.03) | 0.442 f |
| 4 (6.25) | 16 (5.79) | 0.817 f |
| Body Mass Index (kg/m2) [mean (SD)] | 20.97 (0.25) | 25.35 (0.18) | <0.001 * |
| MNA score | |||
| 21 (32.81) | 11 (4.19) | <0.001 f |
| 43 (67.19) | 265 (96.01) | |
| Height-changing score | |||
| [mean (SD)] | |||
| |||
| 3.98 (0.29) | 5.34 (0.36) | 0.037 * |
| 3.49 (0.39) | 4.19 (0.92) | |
| 4.25 (0.57) | 7.75 (1.35) | |
| 4.44 (0.98) | 5.15 (0.84) | |
| 4.65 (1.89) | 6.91 (1.13) | |
| |||
| 2.33 (0.41) | 4.59 (0.72) | 0.019 * |
| 1.96 (0.50) | 3.40 (1.89) | |
| 2.88 (0.88) | 4.36 (0.96) | |
| 2.64 (1.59) | 4.13 (1.77) | |
| 3.25 (1.66) | 5.13 (1.53) | |
| Glucose (g%) | 102.70 (1.19) | 102.19 (2.65) | 0.848 t |
| Albumin (g%) | 4.48 (0.05) | 4.41 (0.11) | 0.303 t |
| Globulin (g%) | 3.39 (0.24) | 3.23 (0.16) | 0.673 t |
| Lymphocyte count (per µL) | 2491.36 (117.80) | 2051.26 (222.21) | 0.007 * |
| Cholesterol (mg%) | 178.05 (5.97) | 205.14 (14.56) | 0.817 t |
| High-density lipoprotein (mg%) | 54.14 (2.17) | 59.13 (3.36) | 0.788 t |
| Low-density lipoprotein (mg%) | 114.01 (5.22) | 131.947 (14.08) | 0.901 t |
| WBC (cell/uL) | 6631.78 (103.38) | 6184.69 (202.03) | 0.052 t |
| Sex of Participants | Mid-Arm Circumference † | Calf Circumference † | Risk of Sarcopenia ‡ |
|---|---|---|---|
| Female (n = 205) Height-changing score | ρ = −0.016 p = 0.816 | ρ = −0.201 p = 0.004 * | Pearson correlation = 0.147 p = 0.035 * |
| Male (n = 135) Height-changing score | ρ = −0.136 p = 0.115 | ρ = −0.261 p = 0.002 * | Pearson correlation = 0.202 p = 0.019 * |
| Height-changing score (both male and female) | ρ = −0.073 p = 0.180 | ρ = −0.233 p < 0.001 * | Pearson correlation = 0.154 p = 0.004 * |
| Risk of Sarcopenia | ||||
|---|---|---|---|---|
| Odd Ratio | 95% Confident Interval | Standard Error | p-Value | |
| Model 1 | ||||
| - Height-hanging score | 1.137 | 1.016, 1.274 | 0.058 | 0.025 |
| - Income | −1.933 | 0.271, 0.986 | 0.329 | 0.045 |
| - BMI | −0.454 | 0.369, 0.559 | 0.106 | <0.001 |
| Model 2 | ||||
| - Height-changing score | 1.146 | 1.021, 1.286 | 0.059 | 0.021 |
| - Income | −1.826 | 0.285, 1.051 | 0.333 | 0.070 |
| - BMI | −2.099 | 0.386, 0.587 | 0.107 | <0.001 |
| - MNA score | −1.443 | 0.555, 0.866 | 0.113 | 0.001 |
| Biomarkers | Lymphocyte Count | WBC | Albumin | Globulin | Cholesterol | HDL-C | LDL-C |
|---|---|---|---|---|---|---|---|
| calf circumference | ρ = 0.169 | ρ = 0.144 | ρ = 0.029 | ρ = 0.037 | ρ = −0.039 | ρ = −0.131 | ρ = 0.008 |
| p = 0.002 * | p = 0.008 * | p = 0.591 | p = 0.788 | p = 0.472 | p = 0.016 * | p = 0.882 | |
| mid-arm circumference | ρ = 0.246 | ρ = 0.259 | ρ = 0.006 | ρ = 0.124 | ρ = −0.074 | ρ = −0.201 | ρ = −0.004 |
| p = <0.001 * | p = <0.001 * | p = 0.906 | p = 0.366 | p = 0.177 | p = <0.001 * | p = 0.938 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikrajang, S.; Komolsuradej, N. Association between Height-Changing Scores and Risk of Sarcopenia Estimated from Anthropometric Measurements in Older Adults: A Cross-Sectional Study. Healthcare 2024, 12, 1005. https://doi.org/10.3390/healthcare12101005
Srikrajang S, Komolsuradej N. Association between Height-Changing Scores and Risk of Sarcopenia Estimated from Anthropometric Measurements in Older Adults: A Cross-Sectional Study. Healthcare. 2024; 12(10):1005. https://doi.org/10.3390/healthcare12101005
Chicago/Turabian StyleSrikrajang, Siwaluk, and Narucha Komolsuradej. 2024. "Association between Height-Changing Scores and Risk of Sarcopenia Estimated from Anthropometric Measurements in Older Adults: A Cross-Sectional Study" Healthcare 12, no. 10: 1005. https://doi.org/10.3390/healthcare12101005
APA StyleSrikrajang, S., & Komolsuradej, N. (2024). Association between Height-Changing Scores and Risk of Sarcopenia Estimated from Anthropometric Measurements in Older Adults: A Cross-Sectional Study. Healthcare, 12(10), 1005. https://doi.org/10.3390/healthcare12101005







