Modeling Nitrogen Fate and Water and Nitrogen Use Efficiencies under Different Greenhouse Vegetable Production Systems Using the WHCNS-Veg Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experiment Design
2.3. Sampling and Analysis
2.4. WHCNS-Veg Model
2.5. Model Evaluation Statistics
2.6. Data Analysis and Calculation
3. Results
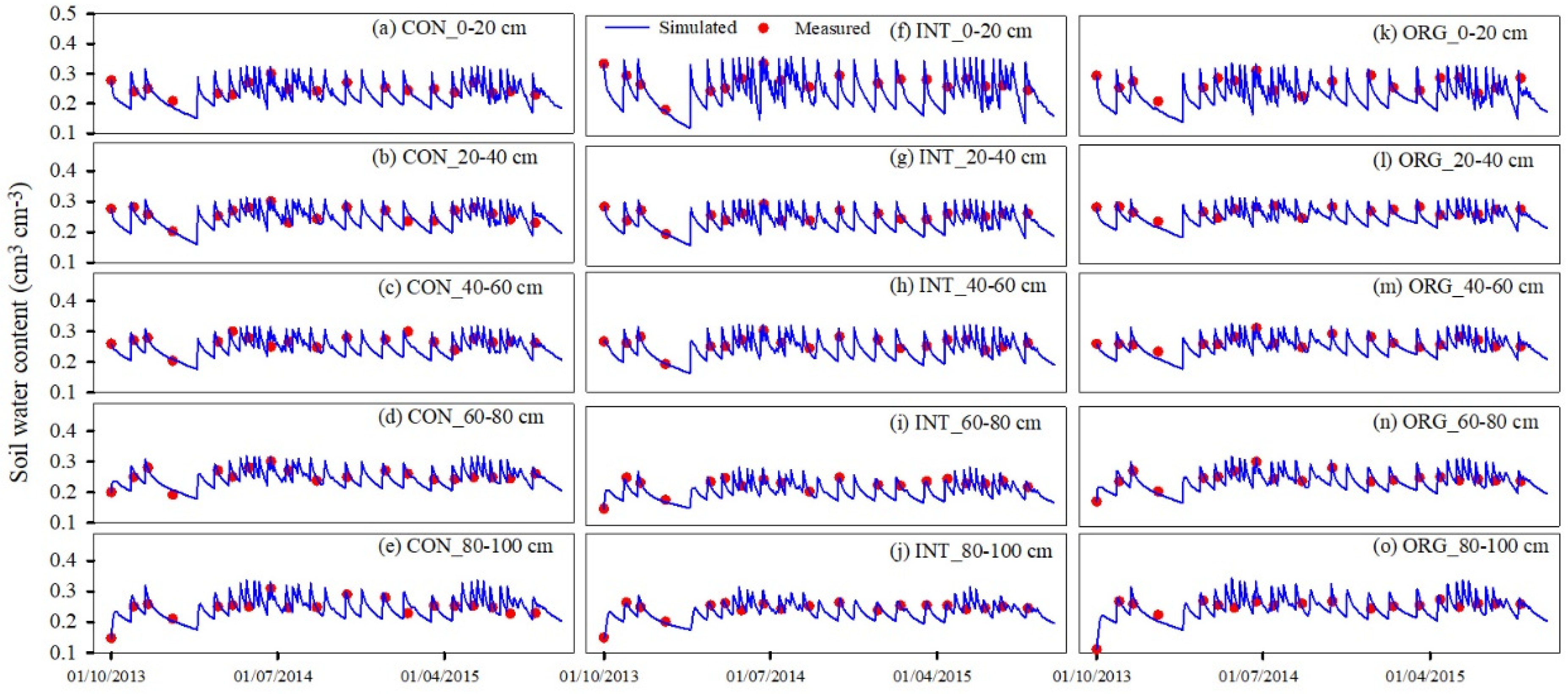
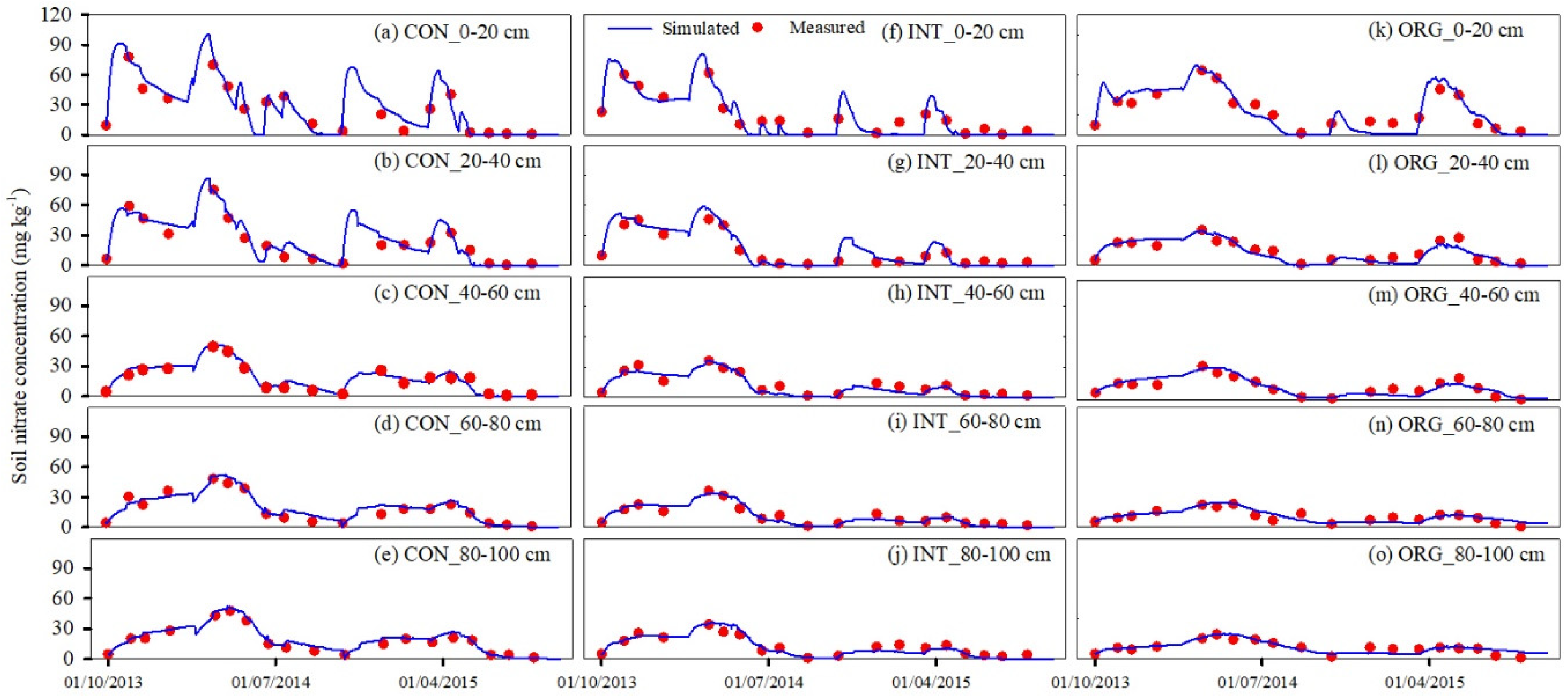
3.1. Model Calibration and Validation
3.1.1. Soil Water and Nitrate Content
3.1.2. Vegetable Yield and N Uptake
3.2. Dynamics of Soil Water Drainage and Nitrate Leaching
3.3. Water Balance and WUE under Three Production Systems
3.4. N Fates and NUE under Three Production Systems
4. Discussion
4.1. The Effects of Production Systems on Vegetable Yield and N Uptake
4.2. The Effects of Production Systems on Nitrate Leaching
4.3. The Effects of Production Systems on Gaseous N Loss
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistics Press: Beijing, China, 2021; (In Chinese with English Abstract). [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Database-Resources. In FAO Statistical Yearbook 2017: World Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; Available online: http://faostat.fao.org (accessed on 4 May 2017).
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, Y.; Shi, X.; Chen, X. Nitrate leaching from open-field and greenhouse vegetable systems in China: A meta-analysis. Environ. Sci. Pollut. Res. 2018, 25, 31007–31016. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, W.; Zhang, Y.; Shi, X.; Chen, X. Nitrous oxide emissions in Chinese vegetable systems: A meta-analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, G.; Yue, S.; Wu, L.; Zhang, W.; Zhang, F.; Chen, X. Closing the N-use efficiency gap to achieve food and environmental security. Environ. Sci. Technol. 2014, 48, 5780–5787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Butterbach-Bahl, K. Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 2014, 374, 977–991. [Google Scholar] [CrossRef]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global greenhouse vegetable production systems are hotspots of soil N2O emissions and nitrogen leaching: A meta-analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ju, X.; Liu, C.; Kou, C. A study on nitrate contamination of ground water sources in areas of protected vegetables-growing fields—A case study in Huimin county, shandong province. Sci. Agric. Sin. 2010, 43, 4427–4436, (In Chinese with English Abstract). [Google Scholar]
- Pang, Z.; Yuan, L.; Huang, T.; Kong, Y.; Liu, J.; Li, Y. Impacts of human activities on the occurrence of groundwater nitrate in an alluvial plain: A multiple isotopic tracers approach. J. Earth Sci. 2013, 24, 111–124. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, W.; Dong, J.; He, S.; Chen, F.; Bi, M.; Liang, B. Irrigation Amount Dominates Soil Mineral Nitrogen Leaching in Plastic Shed Vegetable Production Systems. Agric. Ecosyst. Environ. 2021, 317, 107474. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zheng, C.; Cao, Z. Effects of organic, low input, conventional management practices on soil nematode community under greenhouse conditions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 360–371. [Google Scholar] [CrossRef]
- Audun, K. Relations between nitrogen leaching and food productivity in organic and conventional cropping systems in a long-term field study. Agric. Ecosyst. Environ. 2008, 127, 177–188. [Google Scholar]
- Benoit, M.; Garnier, J.; Billen, G.; Tournebize, J.; Gréhan, E.; Mary, B. Nitrous oxide emissions and nitrate leaching in an organic and a conventional cropping system (seine basin, france). Agric. Ecosyst. Environ. 2015, 213, 131–141. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, P.; Ni, X.; Xiao, Q.; Cao, J.; Zou, G.; Liu, B. Effects of controlled release fertilizer on soil nitrate leaching, N2O emission and fruit yield and quality in greenhouse tomato production system. J. Agro-Environ. Sci. 2014, 33, 1849–1857, (In Chinese with English Abstract). [Google Scholar]
- Greenwood, D.J. Modeling N-response of field vegetable crops grown under diverse conditions with N_ABLE: A review. J. Plant Nutr. 2001, 24, 1799–1815. [Google Scholar] [CrossRef]
- Nendel, C. Evaluation of best management practices for n fertilisation in regional field vegetable production with a small-scale simulation model. Eur. J. Agron. 2009, 30, 110–118. [Google Scholar] [CrossRef]
- Rahn, C.R.; Zhang, K.; Lillywhite, R.; Ramos, C.; Doltra, J.; Paz, J.M.D.; Riley, H.; Fink, M.; Nendel, C.; Thorup-Kristensen, K.; et al. EU-Rotate_N—A decision support system–to predict environmental and economic consequences of the management of nitrogen fertiliser in crop rotations. Eur. J. Hortic. Sci. 2010, 75, 20–32. [Google Scholar]
- Fink, M.; Scharpf, H.C. N-Expert-A Decision Support System for Vegetable Fertilization in the Field: Workshop on Ecological Aspects of Vegetable Fertilization in Integrated Crop Production in the Field. ISHS Acta Hortic. 1992, 339, 67–74. [Google Scholar]
- Rahn, C.; Mead, A.; Draycott, A.; Lillywhite, R.; Salo, T. A sensitivity analysis of the prediction of the nitrogen fertilizer requirement of cauliflower crops using the HRI WELL_N computer model. J. Agric. Sci. 2001, 137, 55–69. [Google Scholar] [CrossRef]
- Zhang, K.; Greenwood, D.J.; White, P.J.; Burns, I.G. A dynamic model for the combined effects of N, P and K fertilizers on yield and mineral composition; description and experimental test. Plant Soil. 2007, 298, 81–98. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, D.; Greenwood, D.J.; Rahn, C.R.; Thorup-Kristensen, K. Development and critical evaluation of a generic 2-D agro-hydrological model (SMCR_N) for the responses of crop yield and nitrogen composition to nitrogen fertilizer. Agric. Ecosyst. Environ. 2009, 132, 160–172. [Google Scholar] [CrossRef]
- Guo, R.; Nendel, C.; Rahn, C.; Jiang, C.; Chen, Q. Tracking nitrogen losses in a greenhouse crop rotation experiment in North China using the EU-Rotate_N simulation model. Environ. Pollut. 2010, 158, 2218–2229. [Google Scholar] [CrossRef]
- Soto, F.; Gallardo, M.; Giménez, C.; Peña-Fleitas, T.; Thompson, R.B. Simulation of tomato growth, water and N dynamics using the EU-Rotate_N model in Mediterranean greenhouses with drip irrigation and fertigation. Agric. Water Manag. 2014, 132, 46–59. [Google Scholar] [CrossRef]
- Liang, H.; Hu, K.; Batchelor, W.D.; Qin, W.; Li, B. Developing a water and nitrogen management model for greenhouse vegetable production in China: Sensitivity analysis and evaluation. Ecol. Model. 2018, 367, 24–33. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Ma, L.; Chadwick, D.; Chen, X. Combined applications of organic and synthetic nitrogen fertilizers for improving crop yield and reducing reactive nitrogen losses from China’s vegetable systems: A meta-analysis. Environ. Pollut. 2021, 269, 116143. [Google Scholar] [CrossRef]
- Wei, Z.; Hoffland, E.; Zhuang, M.; Hellegers, P.; Cui, Z. Organic inputs to reduce nitrogen export via leaching and runoff: A global meta-analysis. Environ. Pollut. 2021, 291, 118176. [Google Scholar] [CrossRef]
- Mitran, T.; Mani, P.K. Effect of organic amendments on rice yield trend, phosphorus use efficiency, uptake, and apparent balance in soil under long-term rice-wheat rotation. J. Plant Nutr. 2016, 40, 1312–1322. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Z.; You, Z.; Yi, X.; Ni, K.; Guo, S.; Ruan, J. Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Jiang, H.; Han, X.; Zou, W.; Hao, X.; Zhang, B. Seasonal and long-term changes in soil physical properties and organic carbon fractions as affected by manure application rates in the Mollisol region of Northeast China. Agric. Ecosyst. Environ. 2018, 268, 133–143. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Green, W.H.; Ampt, G.A. Studies on Soil Phyics. J. Agric. Sci. 1911, 4, 1–24. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A Numerical Model for Water and Solute Movement in and Below the Root Zone; United States Department of Agriculture Agricultural Research Service US Salinity Laboratory: Washington, DC, USA, 1987. [Google Scholar]
- Šimůnek, J.; Hopmans, J.W. Modeling compensated root water and nutrient uptake. Ecol. Model. 2009, 220, 505–521. [Google Scholar] [CrossRef]
- Simunek, J.; Saito, H.; Sejna, M.; van Genuchten, M.T. The HYDRUS-1D Software Package for Simulating the One-Dimensional Movement of Water, Heat, and Multiple Solutes in Variably Saturated Media; Version 4.0; U.S. Salinity Laboratory, Agricultural Research Service, U.S. Department of Agriculture, Colorado School of Mines Publishers: Riverside, CA, USA, 2008. [Google Scholar]
- Hansen, S.; Abrahamsen, P.; Petersen, C.T.; Styczen, M.E. Daisy: Model Use, Calibration, and Validation. Trans. ASABE 2012, 55, 1315–1333. [Google Scholar] [CrossRef]
- Freney, J.R.; Leuning, R.; Simpson, J.R.; Denmead, O.T.; Muirhead, W.A. Estimating ammonia volatilization from flooded rice fields by simplified techniques. Soil Sci. Soc. Am. J. 1985, 49, 1049–1054. [Google Scholar] [CrossRef]
- Willmott, C.J. On the validation of models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Van Liew, M.W.; Garbrecht, J. Hydrologic simulation of the Little Washita River experimental watershed using SWAT. J. Am. Water Resour. Assoc. 2003, 39, 413–426. [Google Scholar] [CrossRef]
- Zhang, H.; Batchelor, W.D.; Hu, K.; Liang, H.; Han, H.; Li, J. Simulation of N2O emissions from greenhouse vegetable production under different management systems in North China. Ecol. Model. 2022, 470, 110019. [Google Scholar] [CrossRef]
- Jensen, L.S.; Salo, T.; Palmason, F.; Breland, T.A.; Henriksen, T.M.; Stenberg, B.; Pedersen, A.; Lundström, C.; Esala, M. Influence of biochemical quality on C and N mineralisation from a broad variety of plant materials in soil. Plant Soil. 2005, 273, 307–326. [Google Scholar] [CrossRef]
- Wei, W.; Yan, Y.; Cao, J.; Christie, P.; Zhang, F.; Fan, M. Effects of combined application of organic amendments and fertilizers on crop yield and soil organic matter: An integrated analysis of long-term experiments. Agric. Ecosyst. Environ. 2016, 225, 86–92. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, Y.; Deng, J.; Wang, L. Multiple-year nitrous oxide emissions from a greenhouse vegetable field in China: Effects of nitrogen management. Sci. Total Environ. 2018, 616, 1139–1148. [Google Scholar] [CrossRef]
- Li, C.; Ma, S.; Shao, Y.; Ma, S.; Zhang, L. Effects of long-term organic fertilization on soil microbiologic characteristics, yield and sustainable production of winter wheat. J. Integr. Agric. 2018, 17, 210–219. [Google Scholar] [CrossRef]
- De la Cruz, V.Y.V.; Cheng, W.; Tawaraya, K. Yield gap between organic and conventional farming systems across climate types and sub-types: A meta-analysis. Agric. Syst. 2023, 211, 103732. [Google Scholar] [CrossRef]
- Wang, S.B.; Feng, P.Y.; Batchelor, W.D.; Hu, K.L.; Li, J. Organic farming decreases nitrate leaching but increases dissolved organic nitrogen leaching in greenhouse vegetable production systems. Plant Soil. 2022, 498, 111–124. [Google Scholar] [CrossRef]
- Xia, L.; Lam, S.K.; Yan, X.; Chen, D. How does recycling of livestock manure in agroecosystems affect crop productivity, reactive nitrogen losses, and soil carbon balance? Environ. Sci. Technol. 2017, 51, 7450–7457. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, M.; Shan, N.; Zhao, Q.; Li, H.; Wang, L. Substituting organic manure for compound fertilizer increases yield and decreases NH3 and N2O emissions in an intensive vegetable production systems. Sci. Total Environ. 2019, 670, 1184–1189. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, H.; Chen, F.; Wang, Q.; Li, J.; Chen, Q.; Liang, B. Optimizing nitrogen management reduces mineral nitrogen leaching loss mainly by decreasing water leakage in vegetable fields under plastic-shed greenhouse. Environ. Pollut. 2022, 308, 119616. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, K.; Zhang, L.; Ji, Y.; Qin, W. Exploring optimal catch crops for reducing nitrate leaching in vegetable greenhouse in North China. Agric. Water Manag. 2019, 212, 273–282. [Google Scholar] [CrossRef]
- Wang, D.; Guo, L.; Zheng, L.; Zhang, Y.; Yang, R.; Li, M.; Ma, F.; Zhang, X.; Li, Y. Effects of nitrogen fertilizer and water management practices on nitrogen leaching from a typical open field used for vegetable planting in northern China. Agric. Water Manag. 2019, 213, 913–921. [Google Scholar] [CrossRef]
- Fan, J.; Xiao, J.; Liu, D.; Ye, G.; Luo, J.; Houlbrooke, D.; Laurenson, S.; Yan, J.; Chen, L.; Tian, J.; et al. Effect of application of dairy manure, effluent and inorganic fertilizer on nitrogen leaching in clayey fluvo-aquic soil: A lysimeter study. Sci. Total Environ. 2017, 592, 206–214. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 6-1–6-13. [Google Scholar] [CrossRef]
- Matsushima, M.; Lim, S.S.; Kwak, J.H.; Park, H.J.; Lee, S.I.; Lee, D.S.; Choi, W.J. Interactive effects of synthetic nitrogen fertilizer and composted manure on ammonia volatilization from soils. Plant Soil 2009, 325, 187–196. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, Y.; Huang, X.; Luan, S. High-resolution measurement of ammonia emissions from fertilization of vegetable and rice crops in the pearl river delta region, China. Atmos. Environ. 2013, 65, 1–10. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C.; Carefoot, J.M.; Janzen, H.H.; Ellert, B.H. Nitrous oxide emissions from an irrigated soil as affected by fertilizer and straw management. Nutri. Cycl. Agroecosyst. 2001, 60, 1–8. [Google Scholar] [CrossRef]
- Huang, T.; Yang, H.; Huang, C.; Ju, X. Effect of fertilizer n rates and straw management on yield-scaled nitrous oxide emissions in a maize-wheat double cropping system. Field Crops Res. 2017, 204, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different c:n ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Zou, J. Relationship between nitrous oxide emission and winter wheat production. Biol. Fertil. Soils 2008, 44, 985–989. [Google Scholar] [CrossRef]
- Novoa, R.S.A.; Tejeda, H.R. Evaluation of the N2O emissions from N in plant residues as affected by environmental and management factors. Nutri. Cycl. Agroecosyst. 2006, 75, 29–46. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- He, F.; Jiang, R.; Chen, Q.; Zhang, F.; Su, F. Nitrous oxide emissions from an intensively managed greenhouse vegetable cropping system in Northern China. Environ. Pollut. 2009, 157, 1666–1672. [Google Scholar] [CrossRef]



| Season | Vegetable | Transplanting Date | Harvest Date | Treatment | Organic Fertilizer | Chemical Fertilizer | Total Amount |
|---|---|---|---|---|---|---|---|
| 2013AW | Cauliflower | CON | 235 | 350 | 585 | ||
| 1 October | 3 January 2014 | INT | 399 | 175 | 574 | ||
| ORG | 798 | / | 798 | ||||
| 2014SS | Eggplant | CON | 227 | 992 | 1219 | ||
| 20 February | 6 September | INT | 459 | 496 | 954 | ||
| ORG | 917 | / | 917 | ||||
| 2014AW | Celery | CON | 149 | 350 | 499 | ||
| 19 October | 2 February 2015 | INT | 254 | 175 | 429 | ||
| ORG | 507 | / | 507 | ||||
| 2015SS | Eggplant | CON | 146 | 525 | 671 | ||
| 11 March | 18 September | INT | 325 | 263 | 588 | ||
| ORG | 651 | / | 651 |
| Soil Layer (cm) | Treatment | BD | Particle Fraction (%) | θr | θs | θfc | θwp | Ks | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||||||
| CON | 1.53 | 0.07 | 0.40 | 0.28 | 0.14 | 21.3 | ||||
| 0–20 | INT | 1.24 | 59.7 | 36.9 | 3.4 | 0.08 | 0.42 | 0.30 | 0.15 | 23.7 |
| ORG | 1.13 | 0.09 | 0.43 | 0.32 | 0.16 | 24.8 | ||||
| 20–40 | 1.49 | 10.1 | 75.9 | 14.0 | 0.07 | 0.34 | 0.27 | 0.10 | 16.1 | |
| 40–60 | 1.44 | 10.1 | 77.9 | 12.0 | 0.07 | 0.36 | 0.26 | 0.11 | 18.8 | |
| 60–80 | 1.36 | 14.1 | 71.9 | 14.0 | 0.07 | 0.36 | 0.25 | 0.11 | 24.6 | |
| 80–100 | 1.36 | 6.1 | 85.9 | 8.0 | 0.07 | 0.40 | 0.25 | 0.11 | 28.1 | |
| Treatment | pH | Soil Organic Matter (g kg−1) | Total N (g kg−1) | Nitrate-N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) |
|---|---|---|---|---|---|---|
| CON | 7.73 | 22.4 | 1.8 | 9.96 | 247.2 | 556.0 |
| INT | 7.57 | 27.9 | 2.0 | 24.9 | 319.1 | 560.0 |
| ORG | 7.45 | 46.6 | 2.9 | 9.8 | 552. 5 | 533.7 |
| Groups | Parameters | Description | Vegetable | ||
|---|---|---|---|---|---|
| Cauliflower | Celery | Eggplant | |||
| Tbase | Base temperature (°C) | 5 | 4 | 15 | |
| Tsum | Accumulated available temperature (°C) | 800 | 850 | 1500 | |
| Kini | Crop coefficient in the initial stage (-) | 0.7 | 0.8 | 0.9 | |
| Crop of | Kmid | Crop coefficient in the middle stage (-) | 1.3 | 1.4 | 1.5 |
| parameters | Kend | Crop coefficient at the end stage (-) | 1 | 1.1 | 1.2 |
| Rmax | Maximum root depth (cm) | 20 | 20 | 50 | |
| αDM | Dry matter accumulation empirical constant (t ha−1) | 1 | 1 | 1 | |
| Nmin | Minimum N concentration of plant (%) | 3.3 | 1.5 | 3 | |
| αN | Empirical parameters of critical function (-) | 17 | 15 | 5 | |
| Vn | Maximum nitrification rate (mg L−1 d−1) | 30 | |||
| Kn | Half saturation constant (mg L−1) | 100 | |||
| Parameters of N | Kd | An empirical proportionality factor (mg mg−1) | 1.5 | ||
| transformation | Ad | Empirical coefficient (-) | 0.3 | ||
| parameters | Kv | First-order kinetic constant of volatilization (d−1) | 0.1 | ||
| Rnit | The ratio of N2O produced by the nitrification process (-) | 0.01 | |||
| Rden | The ratio of N2O produced by the denitrification process (-) | 0.5 | |||
| Soil Layers cm | Calibration (CON) | Validation (INT and ORG) | |||||
|---|---|---|---|---|---|---|---|
| NRMSE (%) | NSE | d | NRMSE (%) | NSE | d | ||
| Soil water content | 0–20 | 5.91 | 0.56 | 0.89 | 6.47 | 0.47 | 0.88 |
| 20–40 | 5.65 | 0.60 | 0.88 | 4.19 | 0.62 | 0.90 | |
| 40–60 | 3.65 | 0.78 | 0.94 | 4.83 | 0.62 | 0.90 | |
| 60–80 | 6.36 | 0.59 | 0.89 | 5.27 | 0.79 | 0.95 | |
| 80–100 | 9.17 | 0.50 | 0.89 | 8.34 | 0.55 | 0.91 | |
| Soil nitrate concentration | 0–20 | 27.41 | 0.90 | 0.98 | 25.04 | 0.91 | 0.98 |
| 20–40 | 26.30 | 0.91 | 0.98 | 29.78 | 0.89 | 0.98 | |
| 40–60 | 23.30 | 0.92 | 0.98 | 28.19 | 0.86 | 0.97 | |
| 60–80 | 24.18 | 0.91 | 0.98 | 29.09 | 0.84 | 0.96 | |
| 80–100 | 19.88 | 0.93 | 0.98 | 27.48 | 0.82 | 0.96 | |
| Fresh yield | 10.40 | 0.96 | 0.99 | 4.84 | 0.99 | 1.00 | |
| Vegetable N uptake | 15.29 | 0.92 | 0.98 | 18.38 | 0.75 | 0.93 | |
| Year | Vegetable | Treatment | I (mm) | P (mm) | E (mm) | T (mm) | ET (mm) | D (mm) | Wbal (mm) | Y (t ha−1) | WUE (kg m−3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013AW | CON | 294 | 0 | 12.1 | 70.3 | 82.4 | 145.4 | 66.1 | 11.0 | 13.3 | |
| Cauliflower | INT | 294 | 0 | 12.1 | 70.3 | 82.4 | 164.1 | 47.5 | 12.8 | 15.5 | |
| ORG | 294 | 0 | 12.1 | 70.3 | 82.4 | 137.0 | 74.6 | 17.2 | 20.8 | ||
| 2014SS | CON | 1078 | 309 | 45.4 | 400.3 | 445.7 | 880.3 | 61.8 | 116.4 | 26.1 | |
| Eggplant | INT | 1078 | 309 | 45.4 | 400.2 | 445.6 | 881.1 | 61.1 | 135.3 | 30.4 | |
| ORG | 1078 | 309 | 45.4 | 400.3 | 445.7 | 882.4 | 59.7 | 139.0 | 31.2 | ||
| 2014AW | CON | 392 | 0 | 10.5 | 91.8 | 102.3 | 243.1 | 46.6 | 63.5 | 62.1 | |
| Celery | INT | 392 | 0 | 10.5 | 91.8 | 102.3 | 238.1 | 51.6 | 70.5 | 69.2 | |
| ORG | 392 | 0 | 10.5 | 91.8 | 102.3 | 267.1 | 22.6 | 57.7 | 56.4 | ||
| 2015 SS | CON | 980 | 198 | 41.7 | 367.0 | 408.7 | 735.8 | 33.9 | 120.0 | 29.4 | |
| Eggplant | INT | 980 | 198 | 41.7 | 366.7 | 408.4 | 736.7 | 33.3 | 103.6 | 25.4 | |
| ORG | 980 | 198 | 41.7 | 366.9 | 408.6 | 755.8 | 14.0 | 165.4 | 40.5 |
| Season | Vegetable | Treatment | N Input (kg N ha−1) | N Output (kg N ha−1) | NUE (kg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | I | Nmin | Nup | Nlea | Ngas | ||||
| 2013AW | CON | 350.0 | 27.4 | 253.6 | 148.7 | 117.9 | 71.6 | 32.5 | |
| Cauliflower | INT | 175.0 | 27.4 | 385.5 | 238.3 | 105.7 | 42.3 | 33.1 | |
| ORG | 0.0 | 27.4 | 406.9 | 201.3 | 28.2 | 15.8 | 70.0 | ||
| 2014SS | CON | 991.7 | 74.4 | 33.8 | 406.9 | 884.2 | 187.6 | 78.2 | |
| Eggplant | INT | 495.8 | 74.4 | 141.8 | 400.3 | 515.6 | 105.2 | 132.3 | |
| ORG | 0.0 | 74.4 | 437.6 | 417.4 | 313.2 | 51.5 | 177.5 | ||
| 2014AW | CON | 350.0 | 21.4 | 15.1 | 145.0 | 123.9 | 61.3 | 192.0 | |
| Celery | INT | 175.0 | 21.4 | 49.6 | 151.6 | 50.0 | 29.1 | 306.8 | |
| ORG | 0.0 | 21.4 | 202.5 | 163.6 | 27.5 | 12.2 | 283.8 | ||
| 2015SS | CON | 525.0 | 70.8 | 26.1 | 394.9 | 287.8 | 73.3 | 158.7 | |
| Eggplant | INT | 262.5 | 70.8 | 101.2 | 286.0 | 88.2 | 35.3 | 253.0 | |
| ORG | 0.0 | 70.8 | 509.7 | 423.9 | 137.4 | 57.1 | 267.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Batchelor, W.D.; Hu, K.; Han, H.; Li, J. Modeling Nitrogen Fate and Water and Nitrogen Use Efficiencies under Different Greenhouse Vegetable Production Systems Using the WHCNS-Veg Model. Plants 2024, 13, 1384. https://doi.org/10.3390/plants13101384
Zhang H, Batchelor WD, Hu K, Han H, Li J. Modeling Nitrogen Fate and Water and Nitrogen Use Efficiencies under Different Greenhouse Vegetable Production Systems Using the WHCNS-Veg Model. Plants. 2024; 13(10):1384. https://doi.org/10.3390/plants13101384
Chicago/Turabian StyleZhang, Hongyuan, William D. Batchelor, Kelin Hu, Hui Han, and Ji Li. 2024. "Modeling Nitrogen Fate and Water and Nitrogen Use Efficiencies under Different Greenhouse Vegetable Production Systems Using the WHCNS-Veg Model" Plants 13, no. 10: 1384. https://doi.org/10.3390/plants13101384






