Exploring Chemical Variability in the Essential Oils of the Thymus Genus
Abstract
:1. Introduction
2. Literature Research Methodology
3. Taxonomy and Essential Oil Production of Thyme
3.1. Taxonomy of Thyme
3.2. Essential oil Biosynthesis in Thyme
3.3. Chemical and Biological Properties of Essential Oil Active Compounds
3.3.1. Chemical and Physical Properties of Thymol
3.3.2. Chemical and Physical Properties of Carvacrol
3.3.3. Biological Activities of Thymol and Carvacrol
3.3.4. Chemical Synthesis Methods of Active Compounds: Focus on Thymol
3.4. Authenticity of Thyme Essential Oil
3.4.1. Methods for Detecting Active Compounds in Essential Oils
3.4.2. Regulations and Standards for Thyme Essential Oil
The International Organization for Standardization (ISO)
The European Pharmacopeia (Ph. Eur)
4. Genetic and Ontogenetic Factors
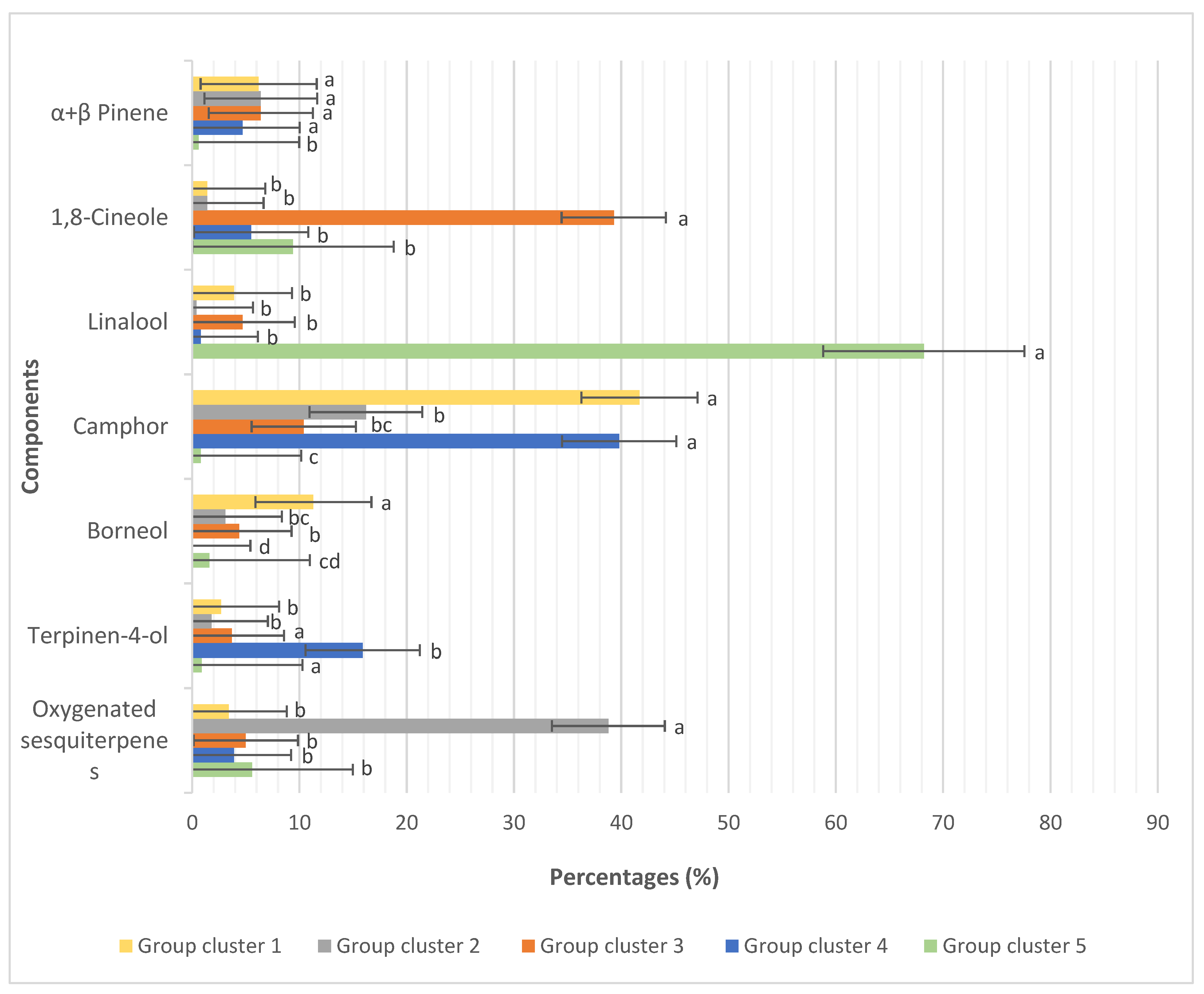
4.1. Chemodiversity between Thymus Taxa
4.2. Intraspecific Variability
4.3. Impact of Phenological Stage
5. Environmental Factors
5.1. Geographical Variation of the Essential Oil Composition
5.2. Climate and Environmental Influences on the Essential Oil Composition
5.2.1. The Effect of Drought Stress
5.2.2. The Effect of Salinity Stress
5.2.3. The Effect of Temperature and Humidity
5.2.4. The Effect of Light
5.2.5. The Effect of Heavy Metals
5.2.6. The Effect of Soil Properties
6. Agrotechnical Factors
6.1. Effect of Biofertilizers
6.2. Effect of Plant Density
7. Impact of Processing Methods
7.1. Drying Methods
7.2. Extraction Methods
7.3. Storage Conditions
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouyahya, A.; Chamkhi, I.; Guaouguaou, F.E.; Benali, T.; Balahbib, A.; El Omari, N.; Taha, D.; El-Shazly, M.; El Menyiy, N. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Food Benefits of Thymus Capitatus. J. Ethnopharmacol. 2020, 259, 112925. [Google Scholar] [CrossRef] [PubMed]
- Mumivand, H.; Shayganfar, A.; Tsaniklidis, G.; Bistgani, Z.E.; Fanourakis, D.; Nicola, S. Pheno-Morphological and Essential Oil Composition Responses to UVA Radiation and Protectants: A Case Study in Three Thymus Species. Horticulturae 2021, 8, 31. [Google Scholar] [CrossRef]
- Sostaric, I.; Arsenijevic, J.; Acic, S.; Stevanovic, Z.D. Essential Oil Polymorphism of Thymus Pannonicus All. (Lamiaceae) in Serbia. J. Essent. Oil Bear. Plants 2012, 15, 237–243. [Google Scholar] [CrossRef]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical Composition, Antioxidant, and Antibiofilm Properties of Essential Oil from Thymus capitatus Plants Organically Cultured on the Greek Island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef] [PubMed]
- Beicu, R.; Neacşu, A.; Imbrea, I.M. Considerations Regarding the Taxonomy of the Genus Thymus in Romania. Res. J. Agric. Sci. 2019, 51, 3–8. [Google Scholar]
- Da Silva, D.V.; Duarte, J.M.; Miguel, M.G.; Leitão, J.M. AFLP Assessment of the Genetic Relationships among 12 Thymus Taxa Occurring in Portugal. Plant Genet. Resour. 2017, 15, 89–92. [Google Scholar] [CrossRef]
- György, Z.; Incze, N.; Pluhár, Z. Differentiating Thymus vulgaris Chemotypes with ISSR Molecular Markers. Biochem. Syst. Ecol. 2020, 92, 104118. [Google Scholar] [CrossRef]
- Tsiftsoglou, O.S.; Stagiopoulou, R.; Krigas, N.; Lazari, D. Exploring the Ecological Preferences and Essential Oil Variability in Wild-Growing Populations of the Endangered Local Greek Endemic Thymus holosericeus (Lamiaceae). Plants 2023, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential Oil Composition of Five Thymus Species from Bulgaria. Chem. Biodivers. 2021, 18, e2100498. [Google Scholar] [CrossRef] [PubMed]
- Ćavar Zeljković, S.; Maksimović, M. Chemical Composition and Bioactivity of Essential Oil from Thymus Species in Balkan Peninsula. Phytochem. Rev. 2015, 14, 335–352. [Google Scholar]
- Stahl-Biskup, E.; Venskutonis, R.P. Thyme. In Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing: Sawston, UK, 2012; Volume 1, pp. 499–525. [Google Scholar] [CrossRef]
- Belmalha, S.; Bouamri, R.; Echchgadda, G.; Ibijbijen, J.; Nassiri, L.; Bachir, S.; Rachidi, F.; Zouhair, R.; Amechrouq, A.; El Idrissi, M. Characterizing the Major Morphological Traits and Chemical Compositions in Nine Species of Wild Thyme from Morocco. Eur. J. Sci. Res. 2017, 145, 188–200. [Google Scholar]
- Morales, R. The History Botany and Taxonomy of the Genus Thymus. In Thyme—The Genus Thymus; Stahl-Biskup, E., Saez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 11–43. [Google Scholar]
- Miguel, G.; Simões, M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Carvalho, L. Composition and Antioxidant Activities of the Essential Oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chem. 2004, 86, 183–188. [Google Scholar] [CrossRef]
- Pinto, E.; Gonçalves, M.J.; Oliveira, P.; Coelho, J.; Cavaleiro, C.; Salgueiro, L. Activity of Thymus caespititius Essential Oil and α-Terpineol against Yeasts and Filamentous Fungi. Ind. Crops Prod. 2014, 62, 107–112. [Google Scholar] [CrossRef]
- Neves, A.; Marto, J.; Duarte, A.; Gonçalves, L.M.; Pinto, P.; Figueiredo, A.C.; Ribeiro, H.M. Characterization of Portuguese Thymbra capitata, Thymus caespititius and Myrtus communis Essential Oils in Topical Formulations. Flavour. Fragr. J. 2017, 32, 392–402. [Google Scholar] [CrossRef]
- Fadli, M.; Bolla, J.M.; Mezrioui, N.E.; Pagès, J.M.; Hassani, L. First Evidence of Antibacterial and Synergistic Effects of Thymus riatarum Essential Oil with Conventional Antibiotics. Ind. Crops Prod. 2014, 61, 370–376. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical Characterization and Antifungal Activities of Four Thymus Species Essential Oils against Postharvest Fungal Pathogens of Citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Girón, V.; Garnatje, T.; Vallès, J.; Pérez-Collazos, E.; Catalán, P.; Valdés, B. Geographical Distribution of Diploid and Tetraploid Cytotypes of Thymus Sect. mastichina (Lamiaceae) in the Iberian Peninsula, Genome Size and Evolutionary Implications. Folia Geobot. 2012, 47, 441–460. [Google Scholar]
- Roxo, M.; Zuzarte, M.; Gonçalves, M.J.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Antifungal and Anti-Inflammatory Potential of the Endangered Aromatic Plant Thymus albicans. Sci. Rep. 2020, 10, 18859. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.R.; Vila, R.; Tomàs, X.; Cañigueral, S.; Da Cunha, A.P.; Adzet, T. Composition and Variability of the Essential Oils of Thymus Species from Section Mastichina from Portugal. Biochem. Syst. Ecol. 1997, 25, 659–672. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. Chemical Characterization and Antibacterial Activity of Thymus moroderi and Thymus piperella Essential Oils, Two Thymus Endemic Species from Southeast of Spain. Food Control. 2012, 27, 294–299. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Barber, X.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. Effect of Chitosan Edible Films Added with Thymus moroderi and Thymus piperella Essential Oil on Shelf-Life of Cooked Cured Ham. J. Food Sci. Technol. 2015, 52, 6493–6501. [Google Scholar] [PubMed]
- Pitarokili, D.; Constantinidis, T.; Saitanis, C.; Tzakou, O. Volatile Compounds in Thymus Sect. teucrioides (Lamiaceae): Intraspecific and Interspecific Diversity, Chemotaxonomic Significance and Exploitation Potential. Chem. Biodivers. 2014, 11, 593–618. [Google Scholar] [CrossRef] [PubMed]
- Benomari, F.Z.; Djabou, N.; Moumani, M.; Hassani, F.; Muselli, A.; Costa, J. Chemical Variability of Essential Oils of Three Subspecies of Thymus munbyanus Boiss. & Reut. from Western Algeria. J. Essent. Oil Res. 2020, 32, 474–484. [Google Scholar] [CrossRef]
- Tefiani, C.; Riazi, A.; Youcefi, F.; Aazza, S.; Gago, C.; Faleiro, M.L.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C.; Megías, C.; et al. Ammoides pusilla (Apiaceae) and Thymus munbyanus (Lamiaceae) from Algeria Essential Oils: Chemical Composition, Antimicrobial, Antioxidant and Antiproliferative Activities. J. Essent. Oil Res. 2015, 27, 131–139. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris Essential Oil: Chemical Composition and Antimicrobial Activity. J. Med. Life 2014, 7, 56. [Google Scholar] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Vouillamoz, J.F.; Christ, B. Thymus vulgaris L.: Thyme. In Medicinal, Aromatic and Stimulant Plants. Handbook of Plant Breeding; Springer: Berlin/Heidelberg, Germany, 2020; Volume 12, pp. 547–557. [Google Scholar] [CrossRef]
- Mokhtarzadeh, S.; Demirci, B.; Khawar, K.M.; Kirimer, N. Determination of Volatile Components in Thymus vulgaris L. under In Vitro Conditions. J. Essent. Oil Bear. Plants 2018, 21, 277–281. [Google Scholar] [CrossRef]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of Chemical Composition of Essential Oils in Wild Populations of Thymus algeriensis Boiss. et Reut., a North African Endemic Species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical Composition and Biological Activities of Algerian Thymus Oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. Essential Oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Yamaura, T.; Tanaka, S.; Tabata, M. Localization of the Biosynthesis and Accumulation of Monoterpenoids in Glandular Trichomes of Thyme. Planta Med. 1992, 58, 153–158. [Google Scholar]
- Turner, G.W.; Gershenzon, J.; Croteau, R.B. Development of Peltate Glandular Trichomes of Peppermint. Plant Physiol. 2000, 124, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Mir, M.; Mett, A.; Belausov, E.; Tal-Meshulam, S.; Frydman, A.; Gidoni, D.; Eya, Y. Peroxisomal Localization of Arabidopsis Isopentenyl Diphosphate Isomerases Suggests That Part of the Plant Isoprenoid Mevalonic Acid Pathway Is Compartmentalized to Peroxisomes. Plant Physiol. 2008, 148, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils: Science, Technology, and Applications; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 39–81. [Google Scholar]
- Sun, M.; Zhang, Y.; Zhu, L.; Liu, N.; Bai, H.; Sun, G.; Zhang, J.; Shi, L. Chromosome-Level Assembly and Analysis of the Thymus Genome Provide Insights into Glandular Secretory Trichome Formation and Monoterpenoid Biosynthesis in Thyme. Plant Commun. 2022, 3, 100413. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The Family of Terpene Synthases in Plants: A Mid-Size Family of Genes for Specialized Metabolism That Is Highly Diversified throughout the Kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E. Essential Oil Chemistry of the Genus Thymus—A Global View. In Thyme—The Genus Thymus; Stahl-Biskup, E., Saez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 75–124. [Google Scholar]
- Agarwal, S.; Tripathi, S.; Mishra, N. Pharmacological Potential of Thymol. Innov. Food Technol. Curr. Perspect. Future Goals 2020, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.J.; Croteau, R. Biosynthesis of Aromatic Monoterpenes: Conversion of γ-Terpinene to p-Cymene and Thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chen, Y.; Fang, J.; Wang, Z.; Xie, C.; Hou, B.; Chen, W.; Xu, F. Solubility and Solution Thermodynamics of Thymol in Six Pure Organic Solvents. J. Chem. Thermodyn. 2016, 92, 198–206. [Google Scholar] [CrossRef]
- Budavari, S.; Maryadele, J.; O’Neil, A.S.; Patricia, E. Heckelman. In The Merck Index; Merck: Rahway, NJ, USA, 1989; pp. 2330–2331. [Google Scholar]
- Ponce Cevallos, P.A.; Buera, M.P.; Elizalde, B.E. Encapsulation of Cinnamon and Thyme Essential Oils Components (Cinnamaldehyde and Thymol) in β-Cyclodextrin: Effect of Interactions with Water on Complex Stability. J. Food Eng. 2010, 99, 70–75. [Google Scholar] [CrossRef]
- Elvers, B. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Török, J.J.; Gömöri, C.; Petkovits, T.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C.; Krisch, J. Anti-Listerial Effect of Selected Essential Oils and Thymol. Acta Biol. Hung. 2016, 67, 333–343. [Google Scholar] [PubMed]
- Natal, C.M.; Fernandes, M.J.G.; Pinto, N.F.S.; Pereira, R.B.; Vieira, T.F.; Rodrigues, A.R.O.; Pereira, D.M.; Sousa, S.F.; Fortes, A.G.; Castanheira, E.M.S.; et al. New Carvacrol and Thymol Derivatives as Potential Insecticides: Synthesis, Biological Activity, Computational Studies and Nanoencapsulation. RSC Adv. 2021, 11, 34024–34035. [Google Scholar] [CrossRef]
- Gandova, V.; Lazarov, A.; Fidan, H.; Dimov, M.; Stankov, S.; Denev, P.; Ercisli, S.; Stoyanova, A.; Gulen, H.; Assouguem, A.; et al. Physicochemical and Biological Properties of Carvacrol. Open Chem. 2023, 21, 20220319. [Google Scholar]
- Kfoury, M.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Determination of Formation Constants and Structural Characterization of Cyclodextrin Inclusion Complexes with Two Phenolic Isomers: Carvacrol and Thymol. Beilstein. J. Org. Chem. 2016, 12, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.R.; Silva, L.P.; Ferreira, O.; Schröder, B.; Coutinho, J.A.P.; Pinho, S.P. Terpenes Solubility in Water and Their Environmental Distribution. J. Mol. Liq. 2017, 241, 996–1002. [Google Scholar] [CrossRef]
- Ali, H.E.; Tong, Y.; Ali, H.E.; Tong, Y. Volatile Oil Concentration and Growth of Thyme (Thymus vulgaris L.) Plants Responded to Red to Blue Light Ratios. Technol. Hortic. 2023, 3, 2. [Google Scholar] [CrossRef]
- Belén Sabater-Jara, A.; Pina Funes, M.; Angeles Pedreño, M.; Belchí-Navarro, S. Essential Oils of Thymbra capitata and Thymus hyemalis and Their Uses Based on Their Bioactivity. In Thymus; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Pancu, D.F.; Buzatu, R.; Milutinovici, R.; Iurciuc, S.; Dolghi, A.; Poenaru, M. Assessment of the Biological Activity of Thyme Essential Oil in the Presence of the Classic Antibiotic Tetracycline. Curr. Health Sci. J. 2022, 48, 18–23. [Google Scholar] [PubMed]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica Subsp. enterica Serovar Typhimurium and Bacillus cereus. Antibiotics 2023, 12, 485. [Google Scholar] [CrossRef]
- Warman, D.J.; Jia, H.; Kato, H. Effects of Thyme (Thymus Vulgaris L.) Essential Oil on Aging-Induced Brain Inflammation and Blood Telomere Attrition in Chronologically Aged C57BL/6J Mice. Antioxidants 2023, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Oubihi, A.; Ballaoui, F.Z.; Imtara, H.; Jaber, H.; Ettouil, A.; Haida, S.; Ouhssine, M.; Noman, O.M.; Mothana, R.A.; Tarayrah, M.; et al. Phytochemical Compounds, Acute Toxicity, Anti-Inflammatory and Antioxidant Activities of Thymus leptobotrys Murb Essential Oil. Molecules 2023, 28, 1355. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Eslamifar, Z.; Nazemi, S.; Hosseini, S.Z.; Behvandi, M.M.; Jafaripour, L. The Effect of Thyme Essential Oil on Liver Injuries Caused by Renal Ischemia-Reperfusion in Rats. Biomed. Res. Int. 2022, 2022, 2988334. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, M.; Iler-Iler, D.; Moreno-Toasa, G.; Rodríguez-Maecker, R.; Arancibia, M.; Iler-Iler, D.; Moreno-Toasa, G.; Rodríguez-Maecker, R. Thyme and Rosemary Essential Oils as an Alternative Control of Plant-Parasitic Nematodes. Management 2017, 256, 2166–2174. [Google Scholar] [CrossRef]
- Akkucuk, S.; Om, K. Can the Thyme Oil Be an Alternative Treatment for Human Demodicosis? Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Noma, Y.; Asakawa, Y. Biotransformation of Monoterpenoids. Compr. Nat. Prod. II Chem. Biol. 2010, 3, 669–801. [Google Scholar] [CrossRef]
- Phillips, M.; Gibbs, H.D. A Synthesis of Thymol from P-Cymene. Ind. Eng. Chem. 1920, 12, 733–734. [Google Scholar]
- Niederl, J.B.; Natelson, S. The Synthesis of Thymol, Chlorothymol and Homologs of Thymol by the Intramolecular Rearrangement of Meta-Cresyl Ethers. J. Am. Chem. Soc. 1932, 54, 1063–1070. [Google Scholar]
- Acevedo, M.D.; Bedogni, G.A.; Okulik, N.B.; Padró, C.L. Study of Gas Phase M-Cresol Alkylation with Methanol on Solid Acid Catalysts. Catal. Lett. 2014, 144, 1946–1954. [Google Scholar]
- Biedermann, W.; Koller, H.; Wedemeyer, K. Process for Preparing Thymol. U.S. Patent US4086283A, 2 June 1976. [Google Scholar]
- Grabowska, H.; Syper, L.; Zawadzki, M. Vapour Phase Alkylation of Ortho-, Meta- and Para-Cresols with Isopropyl Alcohol in the Presence of Sol–Gel Prepared Alumina Catalyst. Appl. Catal. A Gen. 2004, 277, 91–97. [Google Scholar] [CrossRef]
- Ali, A.A.; Gaikar, V.G. Microwave-Assisted Process Intensification of Synthesis of Thymol Using Carbonized Sulfonic Acidic Resin (CSA) Catalyst. Ind. Eng. Chem. Res. 2011, 50, 6543–6555. [Google Scholar] [CrossRef]
- Mesbah, M.; Soltanali, S.; Bahranifard, Z.; Hosseinzadeh, A.; Karami, H. Production of Thymol from Alkylation of M-Cresol with Isopropanol over ZSM-5 Catalysts: Artificial Neural Network (ANN) Modelling. J. Indian Chem. Soc. 2023, 100, 100882. [Google Scholar] [CrossRef]
- Pawar, K.; Sankpal, P.; Patil, S.; Patil, P.; Pawar, A.; Shinde, P. Applications of GC-MS Used in Herbal Plants. Asian J. Pharm. Anal. 2022, 12, 53–55. [Google Scholar] [CrossRef]
- Stashenko, E.; Ren, J. Gas Chromatography-Mass Spectrometry. In Advances in Gas Chromatography; InTech: London, UK, 2014. [Google Scholar] [CrossRef]
- Berkov, S.; Denev, R.; Sidjimova, B.; Zarev, Y.; Shkondrov, A.; Torras-Claveria, L.; Viladomat, F.; Bastida, J. Gas Chromatography–Mass Spectrometry of Some Homolycorine-type Amaryllidaceae Alkaloids. Rapid Commun. Mass Spectrom. 2023, 37, e9506. [Google Scholar] [CrossRef] [PubMed]
- Reuhs, B.L. High-Performance Liquid Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 213–226. [Google Scholar] [CrossRef]
- Heinz, E. Chromatographic Processes. In High Performance Liquid Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–5. [Google Scholar]
- Kulkarni, A.; Nasreen, J.; Seema, N. GC-MS, FT-IR and NMR Spectroscopy Analysis for Metabolome Profiling of Thyme Oil. Asian J. Res. Chem. 2013, 6, 945–949. [Google Scholar]
- ISO 19817:2017; Essential Oil of Thyme [Thymus vulgaris L. and Thymus zygis L.], Thymol Type. International Organization for Standardization: Geneva, Switzerland, 2017.
- 10,000 (01/2023); European Pharmacopoeia 11.0. Council of Europe: Strasbourg, France, 2023.
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential Oil Composition of Ten Species from Sect. Serpyllum of Genus Thymus Growing in Bulgaria. Diversity 2023, 15, 759. [Google Scholar] [CrossRef]
- Aksit, H.; Bayar, Y.; Simsek, S.; Ulutas, Y. Chemical Composition and Antifungal Activities of the Essential Oils of Thymus Species (Thymus pectinatus, Thymus convolutus, Thymus vulgaris) against Plant Pathogens. J. Essent. Oil Bear. Plants 2022, 25, 200–207. [Google Scholar] [CrossRef]
- Kiliç, Ö.; Özdemir, F.A. Essential Oil Composition of Two Thymus kotschyanus Boiss. varietes from Elazığ. Progr. Nutr. 2017, 19, 85–89. [Google Scholar]
- Hanoglu, A.; Hanoglu, D.Y.; Demirci, B.; Yavuz, D.Ö. Chemical Composition of Essential Oil of the Aerial Parts of Wild Growing Thymus capitatus (L.) Hoffm. & Link Species Collected from Three Different Locations in Northern Cyprus. J. Essent. Oil Bear. Plants 2017, 20, 546–551. [Google Scholar] [CrossRef]
- Pluhár, Z.; Sárosi, S.; Pintér, A.; Simkó, H. Essential Oil Polymorphism of Wild Growing Hungarian Thyme (Thymus pannonicus) Populations in the Carpathian Basin. Nat. Prod. Commun. 2010, 5, 1681–1686. [Google Scholar] [CrossRef]
- Jurevičiūtė, R.; Ložienė, K.; Bruno, M.; Maggio, A.; Rosselli, S. Composition of Essential Oil of Lemon Thyme (Thymus × Citriodorus) at Different Hydrodistillation Times. Nat. Prod. Res. 2019, 33, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sowndhararajan, K.; Deepa, P.; Kim, S. Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak. Molecules 2022, 27, 7203. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pawełczak, A.; Rolewska, K.; Węglarz, Z. Morphological and Chemical Traits as Quality Determinants of Common Thyme (Thymus culgaris L.), on the Example of ‘Standard Winter’ Cultivar. Agronomy 2020, 10, 909. [Google Scholar] [CrossRef]
- Antonio Llorens-Molina, J.; Vacas, S.; Burgals Royo, E.; Pilar Santamarina Siurana, M.; Verdeguer Sancho, M. Chemodiversity of Wild Populations of Aromatic Plants as Source of Valuable Essential Oil Profiles. A Study on Thymus culgaris L. from Valencia (Spain). Nat. Volatiles Essent. Oils 2020, 7, 29–50. [Google Scholar]
- Pérez-Sánchez, R.; Ubera, J.L.; Lafont, F.; Gálvez, C. Composition and Variability of the Essential Oil in Thymus zygis from Southern Spain. J. Essent. Oil Res. 2008, 20, 192–200. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Hadian, J.; Nazeri, V. Composition of Essential Oil Compounds from Different Populations of Thymus caramanicus Jalas. J. Appl. Res. Med. Aromat. Plants 2017, 7, 95–98. [Google Scholar] [CrossRef]
- Yousefzadeh, K.; Houshmand, S.; Shiran, B.; Mousavi-Fard, S.; Zeinali, H.; Nikoloudakis, N.; Gheisari, M.M.; Fanourakis, D. Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy 2022, 12, 1008. [Google Scholar] [CrossRef]
- Mumivand, H.; Shayganfar, A.; Hasanvand, F.; Maggi, F.; Alizadeh, A.; Darvishnia, M. Antimicrobial Activity and Chemical Composition of Essential Oil from Thymus daenensis and Thymus fedtschenkoi during Phenological Stages. J. Essent. Oil Bear. Plants 2021, 24, 469–479. [Google Scholar] [CrossRef]
- Shiyab, S.; Shatnawi, M.; Shibli, R.; Alzweiri, M. Influence of Developmental Stage on Yield and Composition of Origanum syriacum L. Oil by Multivariate Analysis. J. Med. Plants Res. 2012, 6, 2985–2994. [Google Scholar] [CrossRef]
- Touhami, A.; Chefrour, A.; Khellaf, N.; Bukhari, A.; Fadel, I. Phytochemical Characterization of the Essential Oils Obtained from Mediterranean Thymus spp. (Lamiacea) Harvested at Different Stages of Growth. J. Pharm. Pharmacol. 2017, 5, 37–45. [Google Scholar] [CrossRef]
- Moisa, C.; Lupitu, A.; Pop, G.; Rodica Chambre, D.; Copolovici, L.; Cioca, G.; Bungau, S.; Maria Copolovici, D. Variation of the Chemical Composition of Thymus culgaris Essential Oils by Phenological Stages. Rev. Chim. 2019, 70, 633–637. [Google Scholar] [CrossRef]
- Toncer, O.; Karaman, S.; Diraz, E.; Sogut, T.; Kizil, S. Essential Oil Composition of Thymus × Citriodorus (Pers.) Schreb. at Different Harvest Stages. Not. Bot. Horti Agrobot. Cluj Napoca 2017, 45, 185–189. [Google Scholar] [CrossRef]
- Llorens-Molina, J.A.; Vacas, S.; Escrivá, N.; Verdeguer, M. Seasonal Variation of Thymus piperella L. Essential Oil Composition. Relationship among γ-Terpinene, p-Cymene and Carvacrol. J. Essent. Oil Res. 2022, 34, 502–513. [Google Scholar] [CrossRef]
- Golparvar, A.R. Effects of Phonological Stages on Quality and Quantity of Essential Oil in Kermanian (Thymus caramanius Jalas). Electron. J. Biol. 2011, 7, 70–73. [Google Scholar]
- Omidbaigi, R.; Sefidkon, F.; Hejazi, M. Essential Oil Composition of Thymus* citriodorus L. Cultivated in Iran. Flavour. Fragr. J. 2005, 20, 237–238. [Google Scholar] [CrossRef]
- Chbel, A.; Elmakssoudi, A.; Rey-Méndez, M.; Barja, J.L.; Filali, O.A.; Soukri, A.; Khalfi, B.E. Comparative Study of Essential Oil Composition, Anti-Bacterial and Antioxidant Activities of the Aerial Parts of Thymus vulgaris Grown in Morocco and France. J. Essent. Oil Bear. Plants 2022, 25, 380–392. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Molina, J.A.; Vacas, S. Effect of Drought Stress on Essential Oil Composition of Thymus vulgaris L. (Chemotype 1, 8-Cineole) from Wild Populations of Eastern Iberian Peninsula. J. Essent. Oil Res. 2017, 29, 145–155. [Google Scholar] [CrossRef]
- Alavi-Samani, S.M.; Kachouei, M.A.; Pirbalouti, A.G. Growth, Yield, Chemical Composition, and Antioxidant Activity of Essential Oils from Two Thyme Species under Foliar Application of Jasmonic Acid and Water Deficit Conditions. Hortic Env. Biotechnol. 2015, 56, 411–420. [Google Scholar]
- Razavizadeh, R.; Farahzadianpoor, F.; Adabavazeh, F.; Komatsu, S. Physiological and Morphological Analyses of Thymus vulgaris L. In Vitro Cultures under Polyethylene Glycol (PEG)-Induced Osmotic Stress. Vitr. Cell. Dev. Biol. -Plant 2019, 55, 342–357. [Google Scholar]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Mohagheghiyan, N. An Investigation of Changes in Antioxidant Enzymes Activities and Secondary Metabolites of Thyme (Thymus vulgaris) Seedlings under In Vitro Salt Stress. J. Plant Biol. Sci. 2015, 7, 41–58. [Google Scholar]
- Hosseini, H.; Mousavi-Fard, S.; Fatehi, F.; Qaderi, A. Changes in Phytochemical and Morpho-Physilogical Traits of Thyme (Thymus vulgaris CV Varico 3) under Different Salinity Levels. J. Med. Plants 2017, 16, 22–33. [Google Scholar]
- Belaqziz, R.; Romane, A.; Abbad, A. Salt Stress Effects on Germination, Growth and Essential Oil Content of an Endemic Thyme Species in Morocco (Thymus maroccanus Ball.). J. Appl. Sci. Res. 2009, 5, 858–863. [Google Scholar]
- Manukyan, A. Secondary Metabolites and Their Antioxidant Capacity of Caucasian Endemic Thyme (Thymus transcaucasicus Ronn.) as Affected by Environmental Stress. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100209. [Google Scholar] [CrossRef]
- Habibi, S.; Qaderi, A.; Fatehi, F. The Study of Relative Expression of Key Genes of Thymol Biosynthesis Pathway in Thymus vulgaris Cv. ‘Varico 3’ under Cold Stress Using Real-Time PCR. J. Med. Plants 2017, 16, 50–58. [Google Scholar]
- Salgado, A.P.S.P.; das Graças Cardoso, M.; de CASTRO, E.M.; Machado, S.M.F.; de Lima Guimarães, L.G.; Andrade, M.A.; Passos, L.O. Caracterização Química e Anatômica de Folhas de Tomilho Provenientes de Plantas Submetidas a Diferentes Condições Luminosas. Biosci. J. 2012, 28, 929–937. [Google Scholar]
- Lalević, D.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Šunić, L.; Kovač, R.; Kovačević, D.; Danilović, B.; Milenković, A.; Stanojević, J.; et al. Shade-Induced Effects on Essential Oil Yield, Chemical Profiling, and Biological Activity in Some Lamiaceae Plants Cultivated in Serbia. Horticulturae 2023, 9, 84. [Google Scholar] [CrossRef]
- Kulbat, K.; Leszczyńska, J. Antioxidants as a Defensive Shield in Thyme (Thymus vulgaris L.) Grown on the Soil Contaminated with Heavy Metals. Biotechnol. Food Sci. 2016, 80, 109–117. [Google Scholar] [CrossRef]
- Figas, A.; Tomaszewska-Sowa, M.; Kobierski, M.; Sawilska, A.K.; Klimkowska, K. Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas. Agriculture 2021, 11, 375. [Google Scholar] [CrossRef]
- Karagözoğlu, Y.; Kıran, R.; Kıran, T.R. Investigation of Heavy Metal Contents in Thyme (Thymus vulgaris) and Ginger (Zingiber Officinale) Sold in Bingöl Herbalists. Middle Black Sea J. Health Sci. 2023, 9, 88–97. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R. Impact of Edaphic and Climatic Factors on Thymus pulegioides Essential Oil Composition and Potential Prevalence of Chemotypes. Plants 2022, 11, 2536. [Google Scholar] [CrossRef] [PubMed]
- Noroozisharaf, A.; Kaviani, M. Effect of Soil Application of Humic Acid on Nutrients Uptake, Essential Oil and Chemical Compositions of Garden Thyme (Thymus vulgaris L.) under Greenhouse Conditions. Physiol. Mol. Biol. Plants 2018, 24, 423–431. [Google Scholar] [PubMed]
- Amiri, F.; Aminzadeh, M.; Abadi, E.A.; Mahdevi, K.; Fadai, S. Factors Affecting on Essential Chemical Composition of Thymus kotschyanus in Iran. World Appl. Sci. J. 2010, 8, 847–856. [Google Scholar]
- Pluhár, Z.; Héthelyi, É.; Kutta, G.; Kamondy, L. Evaluation of Environmental Factors Influencing Essential Oil Quality of Thymus pannonicus All. and Thymus praecox Opiz. J. Herbs. Spices Med. Plants 2007, 13, 23–43. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Ostadi, A.; Alizadeh, K. Improvement in Essential Oil Quantity and Quality of Thyme (Thymus vulgaris L.) by Integrative Application of Chitosan Nanoparticles and Arbuscular Mycorrhizal Fungi under Water Stress Conditions. Plants 2023, 12, 1422. [Google Scholar] [CrossRef] [PubMed]
- Pouramini, P.; Fotokian, M.H.; Dehghan, H.; Hensel, G. Effect of Thiobacillus and Superabsorbent on Essential Oil Components in Thyme Species. J. Essent. Oil Bear. Plants 2019, 22, 799–810. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.S.; Fouda, A.; El-Ela, H.K.A.; El-Ghamry, A.A.; Hassan, S.E.D. Plant Growth-Promoting Properties of Bacterial Endophytes Isolated from Roots of Thymus vulgaris L. and Investigate Their Role as Biofertilizers to Enhance the Essential Oil Contents. Biomol. Concepts 2021, 12, 175–196. [Google Scholar] [PubMed]
- MalekMaleki, F.; Abbasi, N.; Sharifi Ashourabadi, E.; Zare, M.J.; Barary, M. Investigating the Effect of Plant Density on Biochemical Characteristics and Essential Oil in Wild Thyme (Thymbra spicata L.). Plant Prod. 2021, 44, 573–586. [Google Scholar] [CrossRef]
- Kizil, S.; Tonçer, Ö. Effect of Different Planting Densities on Yield and Yield Components of Wild Thyme (Thymbra Spicatavar. spicata). Acta Agron. Hung. 2006, 53, 417–422. [Google Scholar] [CrossRef]
- Punetha, A.; Chauhan, A.; Kumar, D.; KT, V.; Upadhyay, R.K.; Padalia, R.C. Productivity and Essential Oil Quality of Himalayan Thyme (Thymus linearis Benth.) in Relation to Plant Densities and Drying Methods. J. Essent. Oil Res. 2022, 34, 262–269. [Google Scholar] [CrossRef]
- Zrig, A.; Tounekti, T.; AbdElgawad, H.; Hegab, M.M.; Ali, S.O.; Khemira, H. Essential Oils, Amino Acids and Polyphenols Changes in Salt-Stressed Thymus vulgaris Exposed to Open–Field and Shade Enclosure. Ind. Crops Prod. 2016, 91, 223–230. [Google Scholar] [CrossRef]
- Zrig, A.; Ferreira, J.F.S.; Hamouda, F.; Tounekti, T.; Selim, S.; Al Jaouni, S.; Khemira, H.; Abdelgawad, H. The Impact of Foliar Fertilizers on Growth and Biochemical Responses of Thymus vulgaris to Salinity Stress. Arid Land Res. Manag. 2019, 33, 297–320. [Google Scholar] [CrossRef]
- Milenković, L.; Ilić, Z.S.; Šunić, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Modification of Light Intensity Influence Essential Oils Content, Composition and Antioxidant Activity of Thyme, Marjoram and Oregano. Saudi J. Biol. Sci. 2021, 28, 6532–6543. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.A.; Valadabadi, S.A.; Daneshian, J.; Khalvati, M.A. Evaluation Changing of Essential Oil of Balm (Melissa officinalis L.) under Water Deficit Stress Conditions. J. Med. Plants Res. 2009, 3, 329–333. [Google Scholar]
- Akachoud, O.; Bouamama, H.; Facon, N.; Laruelle, F.; Zoubi, B.; Benkebboura, A.; Ghoulam, C.; Qaddoury, A.; Lounès-Hadj Sahraoui, A. Mycorrhizal Inoculation Improves the Quality and Productivity of Essential Oil Distilled from Three Aromatic and Medicinal Plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata. Agronomy 2022, 12, 2223. [Google Scholar] [CrossRef]
- Sadowska, U.; Kopeć, A.; Kourimska, L.; Zarubova, L.; Kloucek, P. The Effect of Drying Methods on the Concentration of Compounds in Sage and Thyme. J. Food Process. Preserv. 2017, 41, e13286. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, Á.A. Effects of Drying Methods on the Composition of Thyme (Thymus vulgaris L.) Essential Oil. Dry. Technol. 2013, 31, 224–235. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Foddai, M.; Del Caro, A.; Desogus, R.; Sanna, I.; Piga, A. Influence of Different Stabilizing Operations and Storage Time on the Composition of Essential Oil of Thyme (Thymus officinalis L.) and Rosemary (Rosmarinus officinalis L.). LWT-Food Sci. Technol. 2011, 44, 244–249. [Google Scholar] [CrossRef]
- Sárosi, S.; Sipos, L.; Kókai, Z.; Pluhár, Z.; Szilvássy, B.; Novák, I. Effect of Different Drying Techniques on the Aroma Profile of Thymus vulgaris Analyzed by GC–MS and Sensory Profile Methods. Ind. Crops. Prod. 2013, 46, 210–216. [Google Scholar] [CrossRef]
- Wesolowska, A.; Grzeszczuk, M.; Jadczak, D. Comparison of the Chemical Composition of Essential Oils Isolated by Water-Steam Distillation and Hydrodistillation from Garden Thyme (Thymus vulgaris L.). J. Essent. Oil Bear. Plants 2016, 19, 832–842. [Google Scholar] [CrossRef]
- Khokhlov, Y.S.; Fedotova, I.A.; Shevchuk, O.M. Changes in the Component Composition of Thymus vulgaris L. Essential Oil Depending on the Method of Distillation. Plant Biol. Hortic. Theory Innov. 2020, 0, 106–115. [Google Scholar] [CrossRef]
- Sadjia, B.; Naima, S.; Chahrazed, B. Extraction of Thyme (Thymus pallecens de Noé) Essential Oil by Steam-Distillation, Steam-Diffusion and Hydro-Distillation Processes: Optimization of Operating Conditions and Antioxidant Activity. J. Essent. Oil Bear. Plants 2012, 15, 336–347. [Google Scholar] [CrossRef]
- Basniwal, P.K.; Shrivastava, P.K.; Dubey, R.; Jain, D. Supercritical Fluid Extraction: A New Milestone in Extraction Technology. Indian J. Pharm. Sci. 2005, 67, 532–539. [Google Scholar]
- Oszagyan, M.; Simandi, B.; Sawinsky, J.; Kery, A.; Lemberkovics, E.; Fekete, J. Supercritical Fluid Extraction of Volatile Compounds from Lavandin and Thyme. Flavour. Fragr. J. 1996, 11, 157–165. [Google Scholar] [CrossRef]
- Kutta, G.; Pluhár, Z.; Sárosi, S. Yield and Composition of Supercritical Fluid Extracts of Different Lamiaceae Herbs. Int. J. Hortic. Sci. 2007, 13, 79–82. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Díaz-Maroto Hidalgo, I.J.; Sánchez-Palomo, E.; Pérez-Coello, M.S. Volatile Components and Key Odorants of Fennel (Foeniculum vulgare Mill.) and Thyme (Thymus vulgaris L.) Oil Extracts Obtained by Simultaneous Distillation−Extraction and Supercritical Fluid Extraction. J. Agric. Food Chem. 2005, 53, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Vas, G.; Vékey, K. Solid-phase Microextraction: A Powerful Sample Preparation Tool Prior to Mass Spectrometric Analysis. J. Mass Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Sárosi, S.; Ruff, J. Optimization of Solid Phase Microextraction Conditions for Analysis of Garden Thyme Volatile Compounds. Kertgazdasag 2013, 45, 75–82. [Google Scholar]
- András, C.; Salamon, B.; György, É.; Mihok, E.; Szép, A. Essential Oil Extraction from Herbs and Their Use in the Food Industry. Acta Agrar. Debreceniensis 2018, 150, 59–74. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata Essential Oils and Plant Extracts for Chemical Composition, Antioxidant, and Antimicrobial Properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Trong, L.V.; Thinh, B.B. Changes in Essential Oil Composition of Thymus vulgaris under Different Storage Conditions and Its Antimicrobial Activity. Proceedings of Universities. Appl. Chem. Biotechnol. 2023, 13, 228–234. [Google Scholar] [CrossRef]
- Mohammadian Yasuj, S.F.; Najafian, S.; Hosseinifarahi, M. Investigating the Storage Conditions of the Essential Oil Compounds of Garden Thyme. J. Med. Plants By-Prod. 2023, 13, 87–94. [Google Scholar] [CrossRef]
- Rowshan, V.; Bahmanzadegan, A.; Saharkhiz, M.J. Influence of Storage Conditions on the Essential Oil Composition of Thymus daenensis Celak. Ind. Crops Prod. 2013, 49, 97–101. [Google Scholar] [CrossRef]

| ISO 19817:2017 | Ph. Eur. 11.0, 10,000 (01/2023) | |||
|---|---|---|---|---|
| Component | Minimum (%) | Maximum (%) | Minimum (%) | Maximum (%) |
| α-Thujene | 0.50 | 1.50 | 0.20 | 1.50 |
| α-Pinene | 0.50 | 2.50 | ns | ns |
| β-Myrcene | 1.00 | 2.80 | 1.00 | 3.00 |
| α-Terpinene | 0.90 | 2.60 | 0.90 | 2.60 |
| γ-Terpinene | 4.00 | 13.00 | 4.00 | 12.00 |
| p-Cymene | 14.00 | 28.00 | 14.00 | 28.00 |
| Linalool | 0.50 | 6.50 | 1.50 | 6.50 |
| Terpinen-4-ol | 0.10 | 2.50 | 0.10 | 2.50 |
| Thymol | 35.00 | 55.00 | 37.00 | 55.00 |
| Carvacrol | 0.50 | 5.50 | 0.50 | 5.50 |
| β-Caryophyllene | 0.50 | 4.00 | ns | ns |
| trans-Sabinene hydrate | <0.01 | 0.50 | ns | ns |
| Carvacrol methyl ether | 0.10 | 1.50 | 0.05 | 1.50 |
| Factor | Reference | Thyme spp. | Treatment | α-Thujene (%) | α-Pinene (%) | Camphene (%) | p-cymene (%) | 1,8-Cineole (%) | α-Terpinene (%) | γ-Terpinene (%) | Linalool (%) | Borneol (%) | α-Terpineol (%) | Thymol (%) | Carvacrol (%) | β-Caryophyllene (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drought | [103] | T. vulgaris | Calus control | 1.70 | 2.20 | 3.00 | 7.70 | 1.40 | 1.50 | 14.20 | 1.20 | 1.80 | 1.00 | 13.20 | 13.50 | 7.20 |
| Callus Polyethylene Glycol (PEG) | 2.00 | 1.60 | 5.10 | 10.80 | 1.60 | 1.10 | 19.30 | 1.10 | 2.10 | 1.30 | 9.80 | 9.60 | 3.10 | |||
| [102] | T. vulgaris T. daenensis | Normal irrigation | 1.08 | 1.32 | 9.92 | 1.34 | 1.95 | 11.33 | 2.00 | 51.99 | 3.80 | 2.37 | ||||
| Slight drought irrigation | 1.10 | 1.17 | 11.79 | 1.19 | 2.05 | 12.66 | 2.35 | 41.46 | 4.77 | 2.71 | ||||||
| Salt | [125] | T. vulgaris | Control | 12.09 | 6.67 | 12.67 | 0.01 | 0.06 | 2.89 | 12.37 | 0.16 | 15.09 | 3.13 | 2.77 | ||
| 150 mM NaCl | 4.15 | 6.06 | 17.35 | 0.05 | 0.08 | 4.75 | 16.45 | 0.38 | 20.06 | 4.48 | 4.10 | |||||
| [126] | T. vulgaris | Control | 1.70 | 2.20 | 6.20 | 15.10 | 1.10 | 22.10 | 5.70 | 29.40 | 3.40 | 2.40 | ||||
| 100 mM NaCl | 2.04 | 1.52 | 3.80 | 8.70 | 0.30 | 24.20 | 10.90 | 32.70 | 1.10 | 1.70 | ||||||
| Light | [127] | T. vulgaris | Unshade (Open field condition) | 0.18 | 0.12 | 0.07 | 2.80 | 0.05 | 0.45 | 3.49 | 0.32 | 0.23 | 0.06 | 8.05 | 0.65 | 0.27 |
| 40% shade | 0.28 | 0.17 | 0.07 | 3.60 | 0.03 | 0.55 | 4.04 | 0.21 | 0.19 | 0.03 | 9.35 | 0.73 | 0.33 | |||
| Temperature | [108] | T. transcaucasicus | 15 °C | 1.82 | 1.14 | 7.10 | 0.88 | 0.06 | 6.34 | 11.70 | 17.37 | 11.18 | 18.05 | 0.02 | ||
| 25 °C | 1.99 | 0.91 | 3.34 | 0.47 | 0.03 | 3.59 | 25.31 | 33.77 | 8.54 | 5.46 | 0.17 | |||||
| Humidity | [108] | T. transcaucasicus | 50% | 1.98 | 0.57 | 1.04 | 0.08 | 1.81 | 6.59 | 42.05 | 14.57 | 2.60 | 14.47 | 0.40 | ||
| 90% | 2.98 | 1.04 | 1.10 | 0.27 | 3.33 | 7.64 | 30.65 | 15.82 | 3.12 | 12.31 | 0.16 | |||||
| Humic acid | [116] | T. vulgaris | Control | 0.23 | 0.25 | 0.23 | 3.46 | 0.58 | 0.35 | 2.24 | 1.73 | 3.12 | 0.17 | 73.08 | 6.18 | |
| 100 gm−2 | 0.26 | 0.27 | 0.25 | 4.24 | 0.53 | 0.48 | 2.40 | 1.58 | 3.42 | 0.12 | 74.15 | 6.20 | ||||
| Arbuscular Mycorrhizal Fungi (AMF) | [129] | T. satureioides | Control | 2.06 | 0.65 | 8.47 | 1.67 | 5.93 | 16.55 | 18.50 | 2.26 | |||||
| AMF+ | 1.27 | 0.46 | 8.37 | 1.62 | 16.73 | 16.75 | 6.05 | 2.57 | ||||||||
| [119] | T. vulgaris | Control | 1.66 | 1.21 | 0.88 | 16.35 | 3.02 | 1.22 | 13.11 | 2.06 | 0.80 | 0.07 | 35.64 | 2.94 | 3.23 | |
| AMF+ | 1.34 | 0.56 | 0.65 | 16.72 | 3.15 | 1.18 | 12.83 | 2.09 | 0.70 | 0.05 | 38.74 | 2.99 | 3.55 | |||
| Plant Growth-Promoting Bacteria (PGPB) | [121] | T. vulgaris | Control | 2.93 | 0.84 | 8.38 | 23.29 | 4.95 | 29.85 | |||||||
| PGPB+ | 3.49 | 1.00 | 7.42 | 22.20 | 1.64 | 33.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etri, K.; Pluhár, Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants 2024, 13, 1375. https://doi.org/10.3390/plants13101375
Etri K, Pluhár Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants. 2024; 13(10):1375. https://doi.org/10.3390/plants13101375
Chicago/Turabian StyleEtri, Karim, and Zsuzsanna Pluhár. 2024. "Exploring Chemical Variability in the Essential Oils of the Thymus Genus" Plants 13, no. 10: 1375. https://doi.org/10.3390/plants13101375






