IPPRAS Cryobank for the Conservation of Orthodox Seeds of Rare, Endangered, Medicinal, and Ornamental Plant Species—Current Research
Abstract
:1. Introduction
2. Results
2.1. Allium Species
2.2. Veratrum Species
2.3. Stipa Species
2.4. Cypripedium calceolus L.
3. Discussion
3.1. Allium Species
3.2. Veratrum Species
3.3. Cypripedium calceolus
3.4. No Correlation Was Found between the Metric Characteristics of Seeds and Their Cryotolerance
3.5. Orthodox Seed Cryopreservation Problems
4. Materials and Methods
4.1. Making the Seed and Fruit Aseptic
4.2. Seed Germination
4.3. Cryopreservation and Thawing of Seeds
4.4. Morphological Characteristics of the Seeds
4.5. Statistics
4.6. Allium Species
4.7. Veratrum Species
4.8. Stipa Species
4.9. Cypripedium calceolus L.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Reed, B.M. Plant cryopreservation: A continuing requirement for food and ecosystem security. Vitr. Cell. Dev. Biol. -Plant 2017, 53, 285–288. [Google Scholar] [CrossRef]
- Panis, B. Sixty Years of Plant Cryopreservation: From Freezing Hardy Mulberry Twigs to Establishing Reference Crop Collections for Future Generations. Acta Hortic. 2019, 1234, 1–8. [Google Scholar] [CrossRef]
- El Merzougui, S.; Benelli, C.; El Boullani, R.; Serghini, M.A. The Cryopreservation of Medicinal and Ornamental Geophytes: Application and Challenges. Plants 2023, 12, 2143. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, A.; Funnekotter, B.; Menon, A.; Phang, P.Y.; Al-Hanbali, A.; Bunn, E.; Mancera, R.L. Current issues in plant cryopreservation. In Current Frontiers in Cryobiology; Katkov, I.I., Ed.; IntechOpen Ltd.: London, UK, 2012; pp. 417–438. [Google Scholar]
- Bajaj, Y.P.S. (Ed.) Cryopreservation of plant cell, tissue, and organ culture for the conservation of germplasm and biodiversity. In Cryopreservation of Plant Germplasm I. Biotechnology in Agriculture and Foresty; Springer: Berlin/Heidelberg, Germany, 1995; Volume 32, pp. 3–28. [Google Scholar]
- Gosling, P.G. Viability Testing. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 2003; pp. 445–481. [Google Scholar]
- Grishchenko, V.I. Results and Perspectives of Cryobiology and Cryomedicine Development. Kriobiologiya 1988, 3, 5–11. [Google Scholar]
- Manuil’skii, V.D. Development of Plant Cryoresistance and Tolerance to Low Temperatures; Naukova Dumka: Kiev, Ukraine, 1992; p. 186. [Google Scholar]
- Tsutsaeva, A.A. Cryobiology and Biotechnology; Naukova Dumka: Kiev, Ukraine, 1987; p. 213. [Google Scholar]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). Role Biotechnol. 2006, 5, 43–54. [Google Scholar]
- Li, D.Z.; Pritchard, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef]
- Vendrame, W.; Faria, R.T.; Sorace, M.; Sahyun, S.A. Orchid cryopreservation. Ciência Agrotecnologia 2014, 38, 213–229. [Google Scholar] [CrossRef]
- Stanwood, P.C. Cryopreservation of seed germplasm for genetic conservation. In Cryopreservation of Plant Cells and Organs; Kartha, K., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 199–225. [Google Scholar]
- Tikhonova, V. L Long-term storage of seeds. Russ. J. Plant Physiol. 1999, 46, 400–408. [Google Scholar]
- Touchell, D.H.; Dixon, K.W. Cryopresevation of seed of Western-Australian native species. Biodivers. Conserv. 1993, 2, 594–602. [Google Scholar] [CrossRef]
- Touchell, D.H.; Dixon, K.W. Cryopresevation for seedbanking of Australian species. Ann. Bot. 1994, 74, 541–546. [Google Scholar] [CrossRef]
- IPPRAS Cryobank. Available online: https://ippras.ru/nauka/nauchnye_podrazdeleniya/gruppa-kriosokhraneniya-rasteniy/kriobank-ifr-ran/ (accessed on 10 February 2024).
- Popov, A.S.; Butenko, R.G.; Glukhova, I.N. Effect of the preparation and deep-freezing conditions on the regeneration of the suspension culture of wild carrot cells. Sov. Plant Physiol. 1978, 25, 972–979. [Google Scholar]
- Yuorieva, N.; Sinetova, M.; Messineva, E.; Kulichenko, I.; Fomenkov, A.; Vysotskaya, O.; Osipova, E.; Baikalova, A.; Prudnikova, O.; Titova, M.; et al. Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology 2023, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.S.; Popova, E.V.; Nikishina, T.V.; Vysotskaya, O.N. Cryobank of plant genetic resources in Russian Academy of Sciences. Int. J. Refrig. 2006, 29, 403–410. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Van Waes, J.M.; Deberg, P.C. In vitro germination of some Western European orchids. Physiol. Plant. 1986, 67, 253–261. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry: Cambridge, UK, 2010; p. 454. ISBN 978-0-85404-190-9. [Google Scholar]
- Ochar, K.; Kim, S.-H. Conservation and Global Distribution of Onion (Allium cepa L.) Germplasm for Agricultural Sustainability. Plants 2023, 12, 3294. [Google Scholar] [CrossRef]
- Kim, H.H.; Popova, E.; Shin, D.J.; Yi, J.Y.; Kim, C.H.; Lee, J.S.; Yoon, M.K.; Engelmann, F. Cryobanking of Korean allium germplasm collections: Results from a 10 year experience. Cryo Lett. 2012, 33, 45–57. [Google Scholar]
- Chojnowski, M.; Wawrzyniak, A.; Burian, M. Polish Collections of Allium; National Institute for Horticultural Research: Skierniewice, Poland. Available online: https://www.ecpgr.cgiar.org/fileadmin/templates/ecpgr.org/upload/WORKING_GROUPS/Allium/10_Allium_collections_in_INHORT_Poland.pdf (accessed on 10 February 2024).
- Senula, A.; Keller, E.R.J. Pollen Cryopreservation to Support Maintenance of a Wild Species Collection of the Genus Allium. Acta Hortic. 2014, 1039, 289–296. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Senula, A. Recent aspects of Allium cryopreservation in the federal German genebank. Acta Hortic. 2016, 1143, 35–44. [Google Scholar] [CrossRef]
- Golubev, F.V. Biological Characteristics of Species of the Genus Allium during Introduction. Ph.D. Thesis, The Tsitsin Main Botanical Garden of Russian Academy of Sciences, Moscow, Russia, 2003. [Google Scholar]
- Nikolaeva, M.G.; Razumova, M.V.; Gladkova, V.N. Reference Book on Dormant Seed Germination; Danilova, M.F., Ed.; Nauka: Leningrad, Russia, 1985; p. 348. (In Russian) [Google Scholar]
- Levitskaya, G.E. The influence of the storage temperature on the seeds of wild species. 3. Seeds with morphological and morphophysiological dormancy. Plant Resour. 2017, 53, 39–50. (In Russian) [Google Scholar]
- Puchalski, J.; Kapler, A.; Niemczyk, M.; Walerowski, P.; Krzyżewski, A.; Nowak, A.; Podyma, W. Long-term seed cryopreservation of rare and endangered Polish Ponto-Panonian plant species. Opole Sci. Soc. Nat. J. 2014, 47, 1–8. [Google Scholar]
- Stanwood, P.C.; Sowa, S. Evaluation of Onion (Allium cepa L.) Seed after 10 Years of Storage at 5, −18, and −196 °C. Seed Physiol. Prod. Technol. 1995, 35, 852–856. [Google Scholar] [CrossRef]
- Lakhanpaul, S.; Babrekar, P.P.; Chandel, K. Seedevaluation after cryopreservation in onion (Allium cepa L.) cultivars. Indian J. Plant Genet. Resour. 1995, 8, 99–105. [Google Scholar]
- Panter, K.K.; Gardner, W.D.; Lee, S. False Hellebore (Veratrum californicum): Historical perspectives and management implications for livestock and wildlife. Society for Range Management: Symposium—Medicinal Uses of Veratrum. In Proceedings of the Annual Meeting with Weed Science Society of America, Denver, CO, USA, 7–11 February 2010. [Google Scholar]
- Berman, D.M.; Karhadkar, S.S.; Hallahan, A.R.; Pritchard, J.I.; Eberhart, C.G.; Watkins, D.N.; Chen, J.K.; Cooper, M.K.; Taipale, J.; Olson, J.M.; et al. Medulloblastoma growth inhibition by Hedgehog Pathway Blockade. Science 2002, 297, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.; Missailidis, S. Targeting the hedgehog pathway: The development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Rev. Med. Chem. 2011, 11, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kou, C.; Ren, Y.; Li, Q.; Wang, T.; Ma, R.; Sun, W.; Xue, Z.; Ma, P. Advances of Veratrum nigrum L. Steroid Alkaloids. Ind. Crops Prod. 2023, 191, 115946. [Google Scholar] [CrossRef]
- Christov, V.; Mikhova, B.; Ivanova, A.; Serly, J.; Molnar, J.; Selenge, D.; Solongo, A.; Kostova, N.; Gerelt-Od, Y.; Dimitrov, D. Steroidal alkaloids of Veratrum lobelianum Bernh. and Veratrum nigrum L. Zeitschrift für Naturforschung C J. Biosci. 2010, 65, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rozhanets, V.V.; Nuzhny, V.P. About permissibility of veratrum intake for conditioned reflex therapy of alcoholism. Narcology 2003, 4, 45–48. (In Russian) [Google Scholar]
- Zheng, B.; Wang, C.; Song, W.; Ye, X.; Xiang, Z. Pharmacokinetics and enterohepatic circulation of jervine, an antitumor steroidal alkaloid from Veratrum nigrum in rats. J. Pharm. Anal. 2019, 9, 367–372. [Google Scholar] [CrossRef]
- Chen, J.; Wen, B.; Wang, Y.; Wu, S.; Zhang, X.; Gu, Y.; Wang, Z.; Wang, J.; Zhang, W.; Yong, J. Jervine exhibits anticancer effects on nasopharyngeal carcinoma through promoting autophagic apoptosis via the blockage of Hedgehog signaling. Biomed. Pharmacother. 2020, 132, 110898. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.-X.; Zhao, Y.-L.; Qin, X.-J.; Yang, X.-W.; Liu, L.; Liu, Y.-P.; Luo, X.-D. Potent anti-inflammatory and analgesic steroidal alkaloids from Veratrum taliense. J. Etnopharmacol. 2016, 179, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Yakan, S.; Aydin, T.; Gulmez, C.; Ozden, O.; Erdogan, K.E.; Daglioglu, Y.K.; Andic, F.; Atakisi, O.; Cakir, A. The protective role of jervine against radiation-induced gastrointestinal toxicity. J. Enzyme. Inhib. Med. Chem. 2019, 34, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Taldaev, A.; Terekhov, R.P.; Melnik, E.V.; Belova, M.V.; Kozin, S.V.; Nedorubov, A.A.; Pomerantseva, T.Y.; Ramenskaya, G.V. Insights into the Cardiotoxic Effects of Veratrum Lobelianum Alkaloids: Pilot Study. Toxins 2022, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Gardens of the Northwest. Available online: https://sadsevzap.ru/rasteniya-dlya-sada/travyanistyie-mnogoletniki/vse-czvetushhie-mnogoletniki/chemericza.html (accessed on 10 February 2024).
- Levitskaya, G.E. Rare species in experimental collection of cryobank wilding seeds in the Institute of Cell Biophysics of RAS. Tambov. Univ. Rep. Ser. Nat. Tech. Sci. 2017, 22, 940–944. (In Russian) [Google Scholar] [CrossRef]
- Ma, R.; Ritala, A.; Oksman-Caldentey, K.-M.; Rischer, H. Development of in vitro Techniques for the Important Medicinal Plant Veratrum californicum. Planta Medica 2006, 72, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- De, L.; Medhi, R. Orchid diversified component of farming systems for profitability and livelihood security of small and marginal farmers. J. Glob. Biosci. 2019, 4, 1393–1406. [Google Scholar]
- Treesuwan, S.; Sritularak, B.; Chanvorachote, P.; Pongrakhananon, V. Cypripedin diminishes an epithelial-to-mesenchymal transition in non-small cell lung cancer cells through suppression of Akt/GSK-3β signalling. Sci. Rep. 2018, 22, 8009. [Google Scholar] [CrossRef] [PubMed]
- Norstog, K. New synthetic medium for the culture of premature barley embryos. In Vitro 1973, 8, 307–308. [Google Scholar] [CrossRef]
- De Pauw, M.A.; Remphrey, W.R. In vitro germination of three Cypripedium species in relation to time of seed collection, media, and cold treatment. Can. J. Bot. 1995, 71, 879–885. [Google Scholar] [CrossRef]
- Klavina, D.; Druva-Lusite, I.; Gailite, A. Asymbiotic Cultivation In Vitro of the Endangered Orchid Cypripedium calceolus L. and Some Aspects of Ex Vitro Growth. Acta Hortic. 2009, 812, 539–554. [Google Scholar] [CrossRef]
- Kozlova, O.N. In vitro culture of temperate orchids at the belarusian centre of plant biochemistry and biotechnology theoretical and applied aspects of plant biochemistry and biotechnology. In Proceedings of the III International Scientific Conference, Minsk, Belarus, 14–16 May 2008; pp. 252–256. [Google Scholar]
- Knudson, L. A new nutrient solution for the germination of orchid seed. Amer. Orchid Soc. Bull. 1946, 15, 214–217. [Google Scholar]
- Jakobsone, G. Germination and development of some terrestrial orchids in vitro. Acta Hortic. 2009, 812, 533–538. [Google Scholar] [CrossRef]
- Znaniecka, J.; Minasiewicz, J. Application of in vitro culture techniques in the conservation of orchids in Gdansk Pomerania. In Proceedings of the XIV Overall Polish In Vitro Culture and Plant Biotechnology Conference Structural, Physiological and Molecular Bases of Plant Differentiation, Poznań, Poland, 14–17 September 2015; p. 142. [Google Scholar]
- Harvais, G. An improved culture medium for growing the orchid Cypripedium reginae axenically. Can. J. Bot. 1982, 60, 2547–2556. [Google Scholar] [CrossRef]
- Chu, C.C.; Mudge, K.W. Propagation and conservation of native Lady’s Slipper Orchids (Cypripedium acaule, C. calceolus, C. reginae). North American terrestrial orchids. Propagation and production. Conference proceedings. In Proceedings of the North American Native Terrestrial Orchid Conference, Germantown, MD, USA, 16–17 March 1996; pp. 107–112. [Google Scholar]
- Malmgren, S. Orchid propagation: Theory and practice. North American terrestrial orchids. Propagation and production. Conference proceedings. In Proceedings of the North American Native Terrestrial Orchid Conference, Germantown, MD, USA, 16–17 March 1996; pp. 63–72. [Google Scholar]
- Konovalova, T.J.; Molkanova, O.I. Cultivating Cypripdium species L. in vitro and pre-cultivating them ex vitro. Probl. Bot. South Sib. Mong. 2020, 19, 15–18. [Google Scholar] [CrossRef]
- Nikishina, T.V.; Vysotskaya, O.N.; Kozlova, O.N.; Levitskaya, G.E. Study of dactylorhiza seeds (D. baltica and D. maculata) from the orchid collection of the Cryobank at Timiryazev Institute of Plant Physiology, Russian Academy of Sciences. Biol. Bull. 2019, 46, 242–250. [Google Scholar] [CrossRef]
- Lavrenko, E.M.; Karamysheva, Z.V. Steppes of the former Soviet Union and Mongolia. In Ecosystems of the World; Coupland, R.T., Ed.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1993; Volume 8b, pp. 3–59. [Google Scholar]
- Osipova, E.S.; Tereshonok, D.V.; Gladkov, E.A.; Evsyukov, S.V.; Stepanova, A.Y. Evaluation of Seed Germination of Six Rare Stipa Species following Low Temperature Stress (Cryopreservation). Life 2023, 13, 2296. [Google Scholar] [CrossRef] [PubMed]
- Nikishina, T.V.; Vysockaja, O.N.; Solov’jova, A.I.; Popov, A.S. Effect of temperature variation on seed viability during cryogenic storage. Plodovod. Jagodovodstvo Ross. 2011, 26, 171–178. (In Russian) [Google Scholar]
- Tikhonova, V.L. Resources of Intraspecific Variability of Wild Herbaceous Plants, Their Study, Conservation and Application. Ph.D. Thesis, All-Russian Research Institute of Ecology, Moscow, Russia, 1992. (In Russian). [Google Scholar]
- Nesterova, S.V. Criopreservation of Wild Plant Seeds of Primorsky Region. Ph.D. Thesis, Botanical Garden-Institute FEB RAS, Vladivostok, Russia, 2004. (In Russian). [Google Scholar]
- De Oliveira, R.S.; Souza, F.V.D.; Dos Santos, I.L.; Souza, S.O.; Aona, L.Y.S.; De Souza, E.H. Cryopreservation and low-temperature storage of seeds of Tillandsia species (Bromeliaceae) with ornamental potential. 3 Biotech 2021, 11, 186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spaeth, S.C. Imbibitional stress and transverse cracking of bean, pea and chickpea cotyledons. HortScience 1986, 21, 110–111. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Manger, K.R.; Prendergast, F.G. Changes in Trifolium arvense seed quality following alternating temperature treatment using liquid nitrogen. Ann. Bot. 1988, 62, 1–11. [Google Scholar] [CrossRef]
- Nikolaeva, M.G. Factors affecting the seed dormancy pattern. In The Physiology and Biochemistry of Seed Development, Dormancyand Germination; Khan, A.A., Ed.; Elsevier Biomedical Press: Amsterdam, The Netherlands, 1982; pp. 51–74. [Google Scholar]
- Ballesteros, D.; Walters, C. Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: Relevance to the physiology of dry biological systems. Plant J. 2011, 68, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Levitskaya, G.E. The influence of the storage temperature on the seeds of wild species. 2. seeds with physiological dormancy in the case of Campanula (Campanulaceae) species. Plant Resour. 2015, 51, 38–51. (In Russian) [Google Scholar]
- Sementsova, M.V. Conservation of rare species of the genus Veratrum L. in the cryobank of IPPRAS. In Proceedings of the International Youth Scientific Forum “Lomonosov-2019”, Lomonosov, Moscow State University, Moscow, Russia, 8–12 April 2019. [Google Scholar]
- Kolomeitseva, G.L.; Nikishina, T.V.; Babosha, A.V.; Ryabchenko, A.S.; Vysotskaya, O.N. Morphophysiology and cryopreservation of seeds of Dendrobium Nobile Lindl. (Orchidaceae) at different stages of development. Acta Physiol. Plant. 2022, 44, 36. [Google Scholar] [CrossRef]
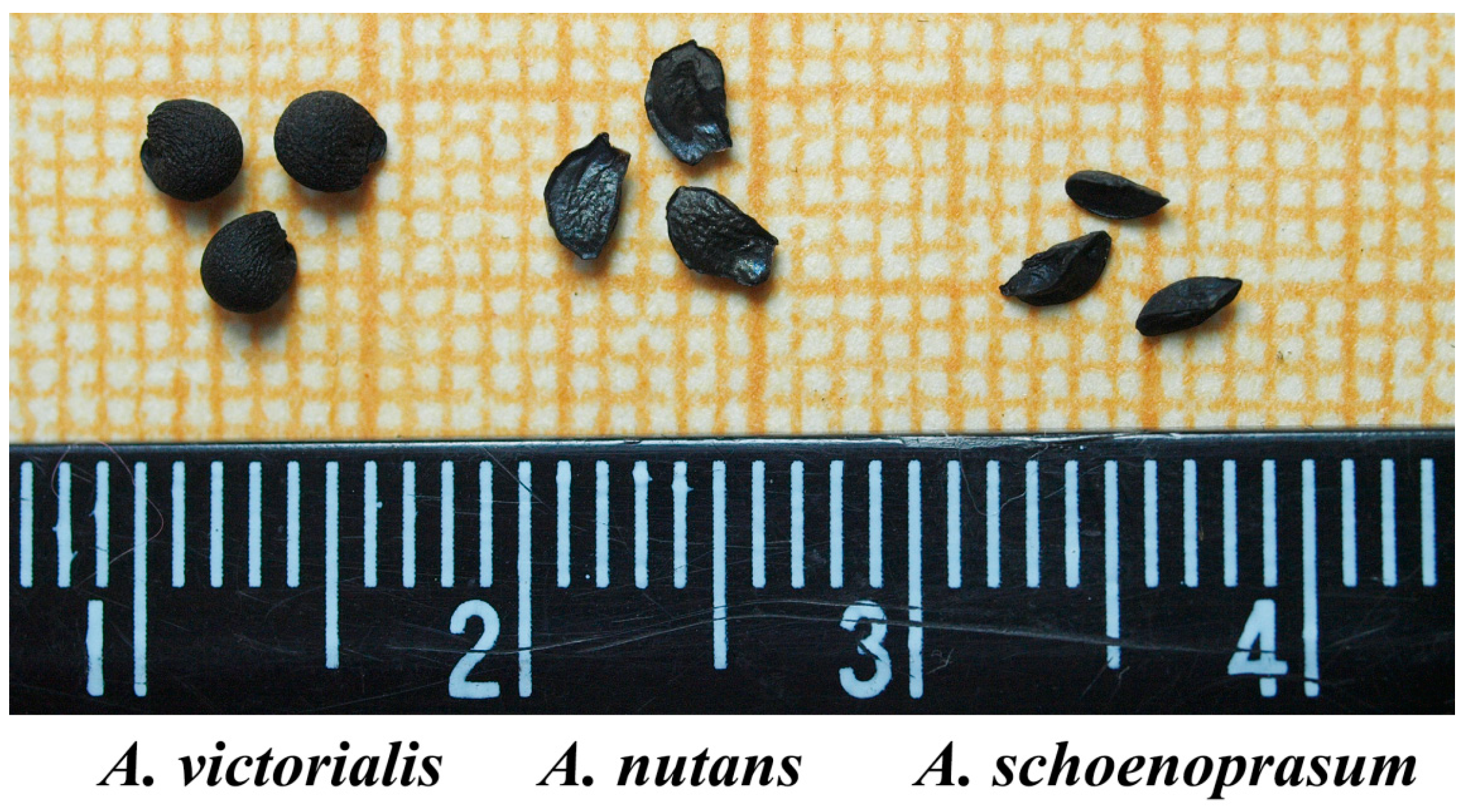
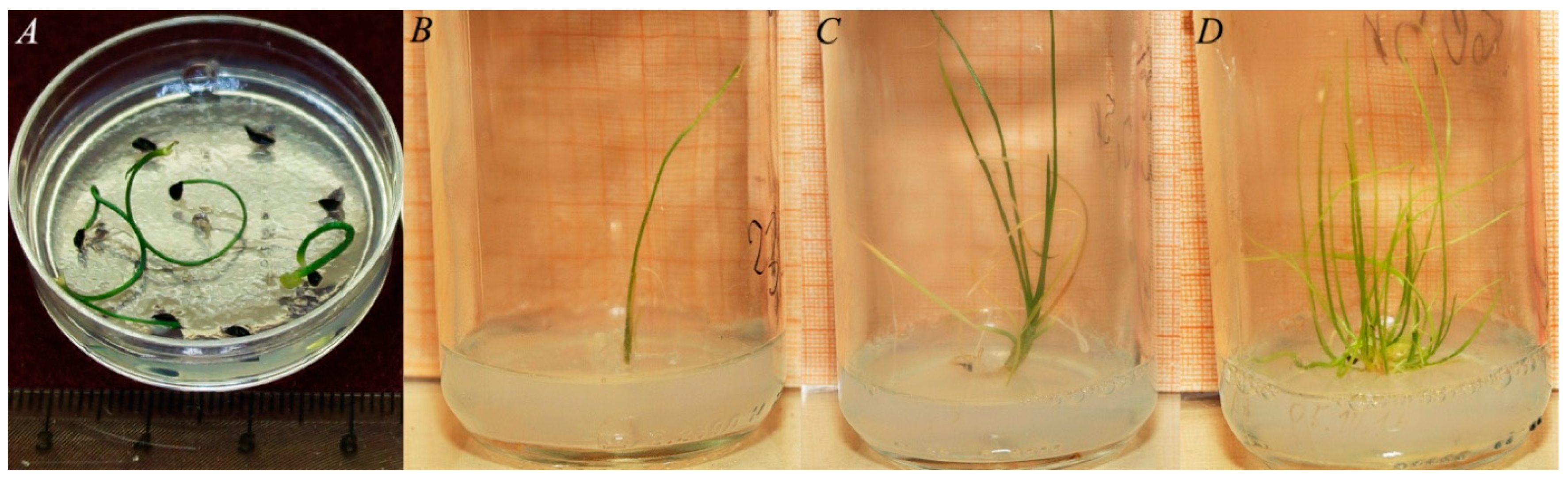
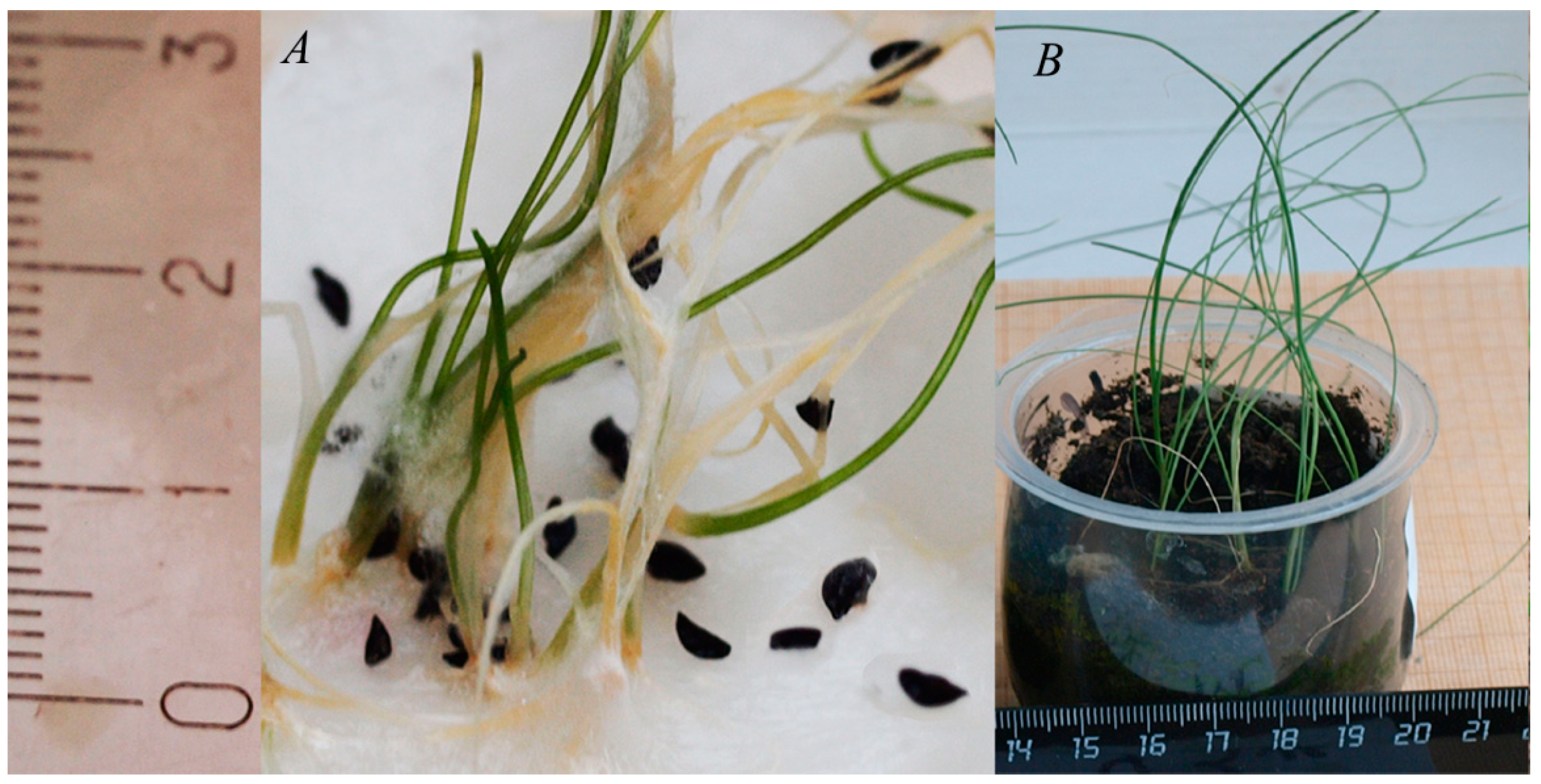



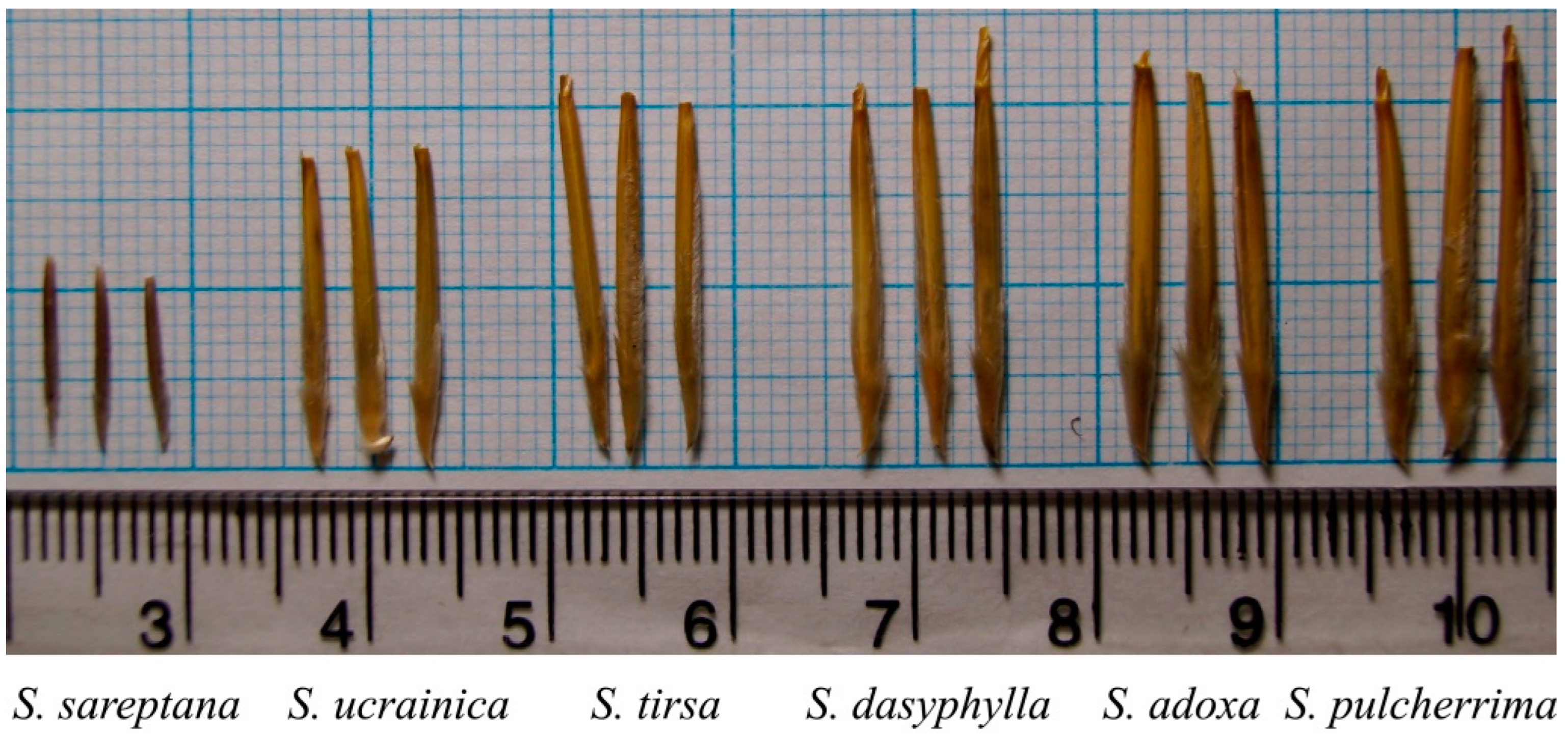

| Species | Length, mm | Diameter, mm | Average Weight of a Seed, mg | Free Water Content (WC), % |
|---|---|---|---|---|
| A. nutans | 3.85 ± 0.15 | 2.11 ± 0.19 | 2.61 ± 0.14 | 7.11 ± 0.48 |
| A.schoenoprasum | 3.05 ± 0.17 | 1.66 ± 0.20 | 1.25 ± 0.11 | 5.24 ± 0.24 |
| A.victorialis | 3.57 ± 0.12 | 3.49 ± 0.13 | 7.82 ± 0.37 | 10.57 ± 0.53 |
| Species | Cryo Date | Thawing Date | Cryo Duration | Germination before Cryo, % | Germination after Cryo, % | Germination Conditions | Mature Plants |
|---|---|---|---|---|---|---|---|
| A. nutans | 20 July 2000 | 5 April 2018 | 17 years 8 months 16 days | 96.55 ± 2.40 a | 50.00 ± 6.93 e | medium | - |
| A.schoenoprasum | 20 July 2000 | 5 April 2018 | 17 years 8 months 16 days | 72.00 ± 6.35 cd | 62.75 ± 6.77 de | medium | - |
| 11 February 2020 | 19 years 6 months 22 days | 72.00 ± 6.35 cd | 60.00 ± 6.93 de | filter paper | 17 | ||
| A.victorialis | 5 June 1989 | 18 March 2020 | 30 years 9 months 13 days | 90.00 ± 4.24 ab | 83.05 ± 4.89 bc | filter paper | 3 |
| Species | Cryo Date | Thawing Date | Cryo Duration | Germination before Cryo, % | Germination after Cryo, % | Germination Conditions | Mature Plants |
|---|---|---|---|---|---|---|---|
| V. nigrum | 20 July 2018 | 23 July 2018 | 3 days | 79.71 ± 4.84 a | 82.69 ± 5.25 a | filter paper | - |
| V. lobelianum | 20 July 2000 | 23 July 2018 | 18 years 3 days | 75.00 ± 6.00 a | 14.81 ± 6.87 b | filter paper | - |
| Species | Length, mm | Diameter, mm | Average Weight of a Seed, mg | Free Water Content (WC), % |
|---|---|---|---|---|
| S. sareptana | 11.05 ± 0.36 | 1.15 ± 0.16 | 3.55 ± 0.11 | 5.13 ± 0.51 |
| S. ucrainica | 17.45 ± 0.41 | 1.70 ± 0.21 | 16.00 ± 0.87 | 6.62 ± 0.49 |
| S. tirsa | 20.85 ± 0.54 | 1.80 ± 0.18 | 22.40 ± 1.46 | 6.77 ± 0.87 |
| S. dasyphylla | 21.10 ± 0.63 | 2.10 ± 0.09 | 23.85 ± 2.03 | 6.94 ± 0.94 |
| S. adoxa | 22.15 ± 0.71 | 2.20 ± 0.11 | 30.65 ± 3.18 | 5.55 ± 0.62 |
| S. pulcherrima | 22.75 ± 0.75 | 2.30 ± 0.11 | 25.40 ± 1.85 | 6.34 ± 0.78 |
| Species | Germination Rates before Cryopreservation, % | Germination Rates after Cryopreservation, % |
|---|---|---|
| Room T (20–25 °C) (6 Months) + Stratification 42 Days | Cryopreservation in Liquid N2 (1951 Days) + Pre-Treatment with NaOH or H2O2 | |
| S. sareptana | 57.14 ± 6.61 b | 90.00 ± 4.24 a (H2O2) |
| S. ucrainica | 43.14 ± 6.94 bc | 7.02 ± 3.38 f (H2O2) |
| S. tirsa | 32.00 ± 6.60 cd | - |
| S. dasyphylla | 28.30 ± 6.19 cd | 11.32 ± 4.35 ef (H2O2) |
| S. adoxa | 21.43 ± 5.48 de | 12.96 ± 4.57 ef (NaOH) |
| S. pulcherrima | 56.14 ± 6.57 b | 6.98 ± 3.88 f (NaOH) |
| Species | Cryo Date | Thawing Date | Cryo Duration | Culture Medium | Protocorm Formation Rate, % |
|---|---|---|---|---|---|
| C. calceolus | 5 December 2022 | 8 December 2022 | 3 days | 1/2 MS | 24.98 ± 1.35 a |
| MS + coconut milk | 10.02 ± 0.89 c | ||||
| BM1 | 15.02 ± 1.11 b |
| Species | Collection Site | Collection Date | Storage Conditions and Duration | Number of Seeds in the Germination Test | |
|---|---|---|---|---|---|
| before Cryo | after Cryo | ||||
| A. nutans | BG RAS (collected by Tihonova V.L.) | 1998 | refrigerator +4 °C, darkness, 2 years | 58 | 52 medium |
| A.schoenoprasum | BG RAS (collected by V.L. Tihonova) | 1998 | refrigerator +4 °C, darkness, 2 years | 50 | 51 medium |
| 50 filter paper | |||||
| A.victorialis | Moscow Region (collected by T.V. Daletskaya) | 1987 | 20–25 °C, 40–60% humidity, darkness, 2 years | 50 | 59 filter paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nits, O.S.; Sementsova, M.V.; Osipova, E.S.; Tereshonok, D.V.; Gladkov, E.A. IPPRAS Cryobank for the Conservation of Orthodox Seeds of Rare, Endangered, Medicinal, and Ornamental Plant Species—Current Research. Plants 2024, 13, 1354. https://doi.org/10.3390/plants13101354
Nits OS, Sementsova MV, Osipova ES, Tereshonok DV, Gladkov EA. IPPRAS Cryobank for the Conservation of Orthodox Seeds of Rare, Endangered, Medicinal, and Ornamental Plant Species—Current Research. Plants. 2024; 13(10):1354. https://doi.org/10.3390/plants13101354
Chicago/Turabian StyleNits, Olga Sergeevna, Mariya Vladimirovna Sementsova, Ekaterina Sergeevna Osipova, Dmitry Viktorovich Tereshonok, and Evgeny Aleksandrovich Gladkov. 2024. "IPPRAS Cryobank for the Conservation of Orthodox Seeds of Rare, Endangered, Medicinal, and Ornamental Plant Species—Current Research" Plants 13, no. 10: 1354. https://doi.org/10.3390/plants13101354






