Two-Dimensional High-Performance Thin-Layer Chromatography with Bioautography for Distinguishing Angelicae Dahuricae Radix Varieties: Chemical Fingerprinting and Antioxidant Profiling
Abstract
:1. Introduction
2. Results
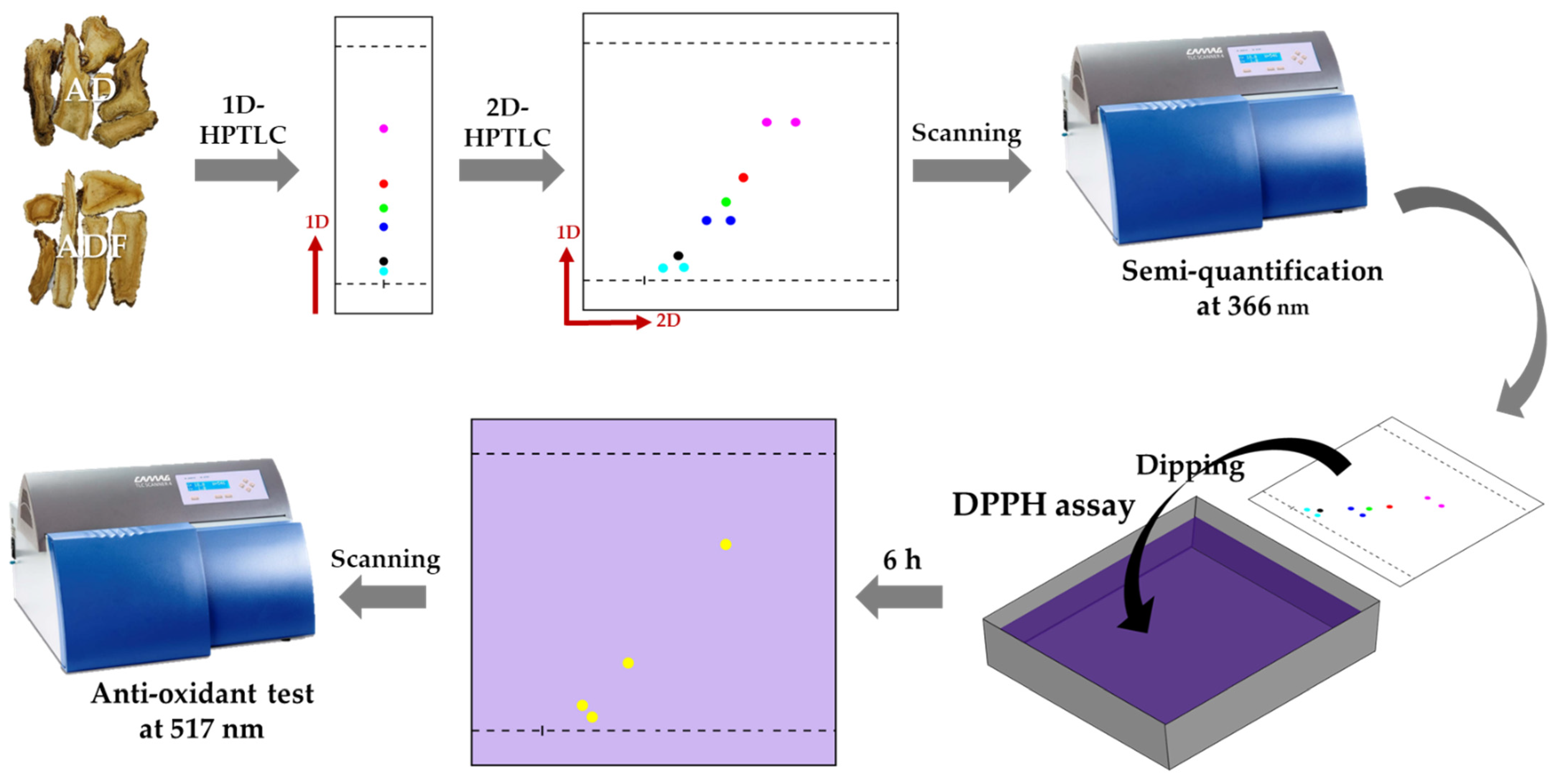
2.1. HPTLC Fingerprints
2.1.1. One-Dimensional HPTLC
2.1.2. Two-Dimensional HPTLC
2.2. Quantitative Analysis of Coumarins
2.3. DPPH Bioautography on 2D-HPTLC
2.4. Identification of the Compounds from 2D-HPTLC and DPPH Bioautography
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. HPTLC Analysis
4.3.1. Equipment
4.3.2. Analytical Conditions for 1D-HPTLC
4.3.3. Analytical Conditions for 2D-HPTLC
4.4. DPPH Bioautography
4.5. MS-Interface Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Chen, Y.; Lin, M.; Wen, J.; Fan, G.; Wu, Y. High-performance liquid chromatographic method for the determination and pharmacokinetic study of oxypeucedanin hydrate and byak-angelicin after oral administration of Angelica dahurica extracts in mongrel dog plasma. J. Pharm. Biomed. Anal. 2007, 44, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Ha, T.K.Q.; Cho, H.M.; An, J.-P.; Kim, S.K.; Kim, C.-S.; Kim, E.; Oh, W.K. Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J. Ethnopharmacol. 2020, 259, 112945. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.; Chen, Y.; Hwang, S.; Chen, T.; Chen, F. The prescriptions frequencies and patterns of Chinese herbal medicine for allergic rhinitis in Taiwan. Allergy 2006, 61, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-Y.; Chao, J.; Chen, P.-Y.; Su, S.-Y.; Lin, C.-Y.; Huang, S.-S. Ethnobotanical survey on skin whitening prescriptions of traditional Chinese medicine in Taiwan. Front. Pharmacol. 2021, 12, 736370. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Chen, J.; Miao, M. Characteristic Analysis of Internal and External Use of Chloasma Based on Data Mining. In Proceedings of the 2019 3rd International Conference on Data Science and Business Analytics (ICDSBA), Istanbul, Turkey, 11–12 October 2019. [Google Scholar]
- Fang, C.-L.; Goswami, D.; Kuo, C.-H.; Day, C.H.; Lin, M.-Y.; Ho, T.-J.; Yang, L.-Y.; Hsieh, D.J.-Y.; Lin, T.-K.; Huang, C.-Y. Angelica dahurica attenuates melanogenesis in B16F0 cells by repressing Wnt/β-catenin signaling. Mol. Cell. Toxicol. 2023, 19, 135–143. [Google Scholar] [CrossRef]
- Liang, W.-H.; Chang, T.-W.; Charng, Y.-C. Effects of drying methods on contents of bioactive compounds and antioxidant activities of Angelica dahurica. Food Sci. Biotechnol. 2018, 27, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J. Oxypeucedanin: Chemotaxonomy, isolation, and bioactivities. Plants 2021, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.M.; Moon, T.C.; Hong, T.G.; Park, K.M.; Son, J.K.; Chang, H.W. 5-Methoxy-8-(2-hydroxy-3-buthoxy-3-methylbutyloxy)-psoralen isolated from Angelica dahurica inhibits cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharmacal Res. 2008, 31, 617–621. [Google Scholar] [CrossRef]
- Li, D.; Wu, L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp. Ther. Med. 2017, 14, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, Y.S.; Ryu, S.Y. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res. 2007, 21, 288–290. [Google Scholar] [CrossRef] [PubMed]
- The Korean Pharmacopoeia, 12th ed.; Korea Ministry of Food and Drug Safety Administration: Seoul, Republic of Korea, 2019.
- Pharmacopoeia of the People’s Republic of China 2020 Edition; Chinese CPC: Beijing, China, 2020.
- Taiwan Herbal Pharmacopeia, 4th ed.; Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare: Taipei, Taiwan, 2022.
- Japanese Pharmacopoeia, 17th ed.; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016.
- The Pharmacopoeia of Democratic People’s Republic of Korea, 8th ed.; Democratic People’s Republic of Korea Pharmacopoeia Commission; Medical Science Publishing House: Pyongyang, Democratic People’s Republic of Korea, 2018.
- Srivastava, M. An overview of HPTLC: A modern analytical technique with excellent potential for automation, optimization, hyphenation, and multidimensional applications. In High-Performance Thin-Layer Chromatography (HPTLC); Springer: Berlin/Hiedelberg, Germany, 2010; pp. 3–24. [Google Scholar] [CrossRef]
- Shewiyo, D.H.; Kaale, E.; Risha, P.; Dejaegher, B.; Smeyers-Verbeke, J.; Vander Heyden, Y. HPTLC methods to assay active ingredients in pharmaceutical formulations: A review of the method development and validation steps. J. Pharm. Biomed. Anal. 2012, 66, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Moon, K.; Woo, W. Two minor furanocoumarins of Angelica dahurica. Arch. Pharmacal Res. 1991, 14, 165. [Google Scholar] [CrossRef]
- Ze-Hui, P.; Hui-Chen, C.; Zhu-Jun, W.; Chang-Qi, Y. The karyotypes of Angelica dahurica and their taxonomical significance (Umbelliferae). J. Syst. Evol. 1985, 23, 185. [Google Scholar]
- Shi, H.; Chang, Y.Q.; Feng, X.; Yang, G.Y.; Zheng, Y.G.; Zheng, Q.; Zhang, L.L.; Zhang, D.; Guo, L. Chemical comparison and discrimination of two plant sources of Angelicae dahuricae Radix, Angelica dahurica and Angelica dahurica var. formosana, by HPLC-Q/TOF-MS and quantitative analysis of multiple components by a single marker. Phytochem. Anal. 2022, 33, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Guo, J.; Li, T.; Zhao, M.; Zou, T.; Song, H.; Alim, A. Comparison and Identification of the Aroma-Active Compounds in the Root of Angelica dahurica. Molecules 2019, 24, 4352. [Google Scholar] [CrossRef] [PubMed]
- Noh, P.; Kim, W.J.; Yang, S.; Park, I.; Moon, B.C. Authentication of the herbal medicine Angelicae Dahuricae Radix using an ITS sequence-based multiplex SCAR assay. Molecules 2018, 23, 2134. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Wang, J.; Zhang, L.; Gao, B.; Shi, S.; Wang, X.; Li, J.; Tu, P. Simultaneous characterisation of fifty coumarins from the roots of Angelica dahurica by Off-line Two-dimensional high-performance liquid chromatography coupled with electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2014, 25, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, Q. Study on the spatial distribution of coumarins in Angelica dahurica root by MALDI-TOF-MSI. Phytochem. Anal. 2023, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lechner, D.; Stavri, M.; Oluwatuyi, M.; Pereda-Miranda, R.; Gibbons, S. The anti-staphylococcal activity of Angelica dahurica (Bai Zhi). Phytochemistry 2004, 65, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Wei, J.; Kong, L.; Song, M.; Zhang, Y.; Xiao, X.; Cao, H.; Jin, Y. Network pharmacology and molecular docking reveal the mechanism of Angelica dahurica against Osteosarcoma. Medicine 2022, 101, e31055. [Google Scholar] [CrossRef] [PubMed]

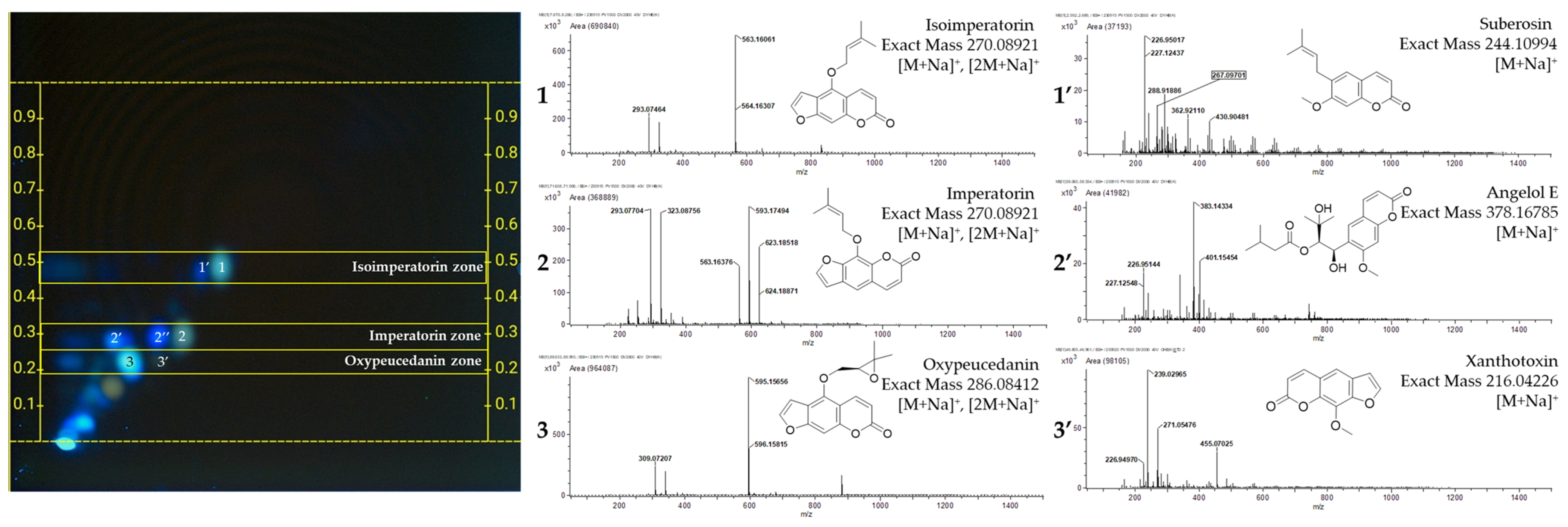

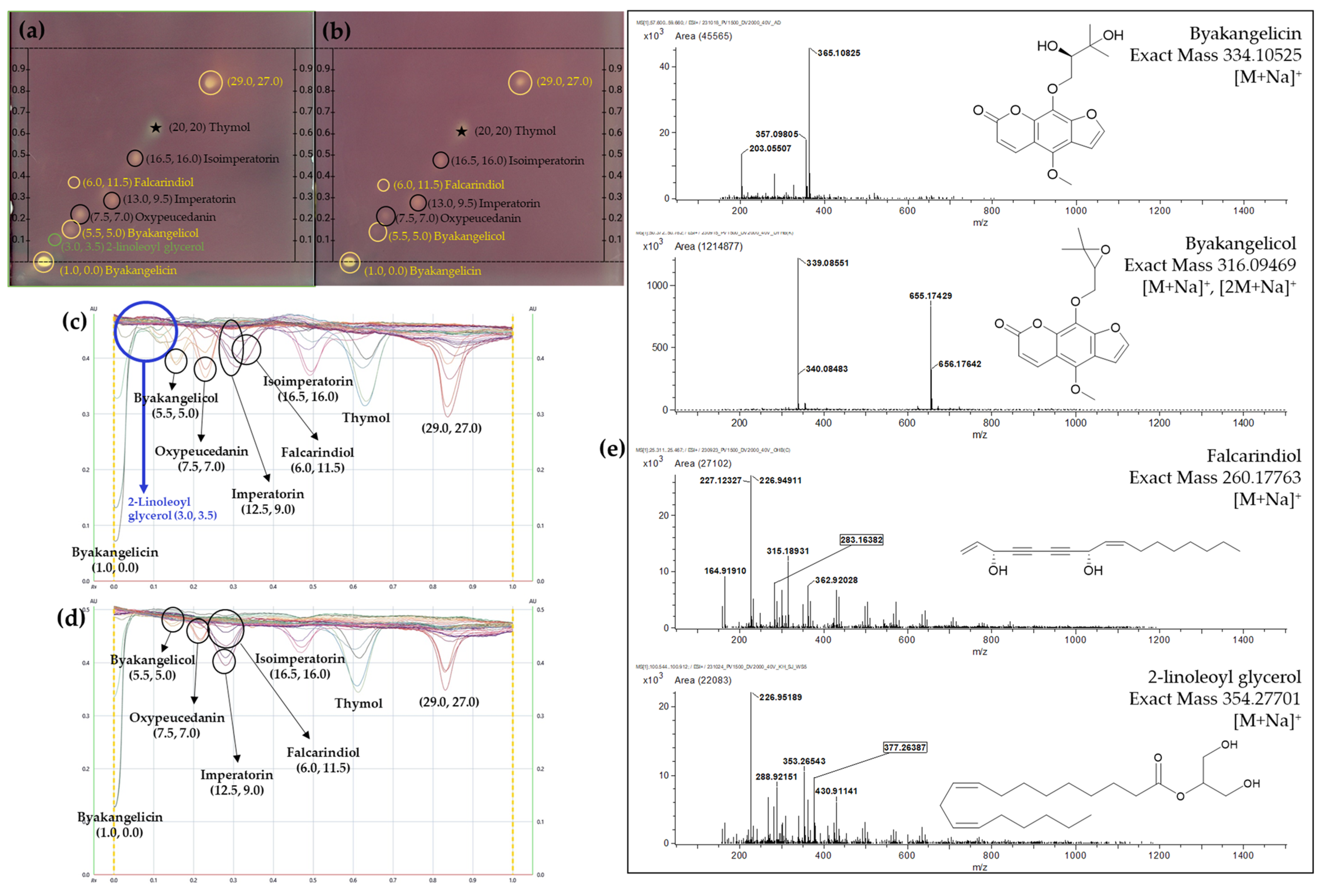
| No. | Position (x, y) | m/z 1 | Quasi-Molecular Ion | Mass Difference (mu) | Molecular Formula | Identification | Ref |
|---|---|---|---|---|---|---|---|
| 1 | (16.5, 16.0) | 293.07464 | [M+Na]+ | −4.34 | C16H14O4 | Isoimperatorin | [24] 2 |
| 1′ | (14.5, 15.5) | 267.09701 | [M+Na]+ | −2.71 | C15H16O3 | Suberosin | [21] 2 |
| 2 | (13.0, 9.5) | 293.07704 | [M+Na]+ | −1.93 | C16H14O4 | Imperatorin | [25] 2 |
| 2′ | (6.5, 9.0) | 401.15454 | [M+Na]+ | −3.08 | C20H26O7 | Angelol E | [25] |
| 3 | (7.5, 7.0) | 309.07207 | [M+Na]+ | −1.82 | C16H14O5 | Oxypeucedanin | [25] 2 |
| 3′ | (10.5, 7.5) | 239.02965 | [M+Na]+ | −2.38 | C12H8O4 | Xanthotoxin | [25] 2 |
| 4 | (6.0, 11.5) | 283.16382 | [M+Na]+ | −3.58 | C17H24O2 | Falcarindiol | [26] 2 |
| 5 | (5.5, 5.0) | 339.08551 | [M+Na]+ | 1.05 | C17H16O6 | Byakangelicol | [25] 2 |
| 6 | (3.0, 3.5) | 377.26387 | [M+Na]+ | −2.91 | C21H38O4 | 2-Linoleoyl glycerol * | [27] 2 |
| 7 | (1.0, 0.0) | 357.09805 | [M+Na]+ | 3.02 | C17H18O7 | Byakangelicin | [24] 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, S.; Park, G.; Jang, Y.P. Two-Dimensional High-Performance Thin-Layer Chromatography with Bioautography for Distinguishing Angelicae Dahuricae Radix Varieties: Chemical Fingerprinting and Antioxidant Profiling. Plants 2024, 13, 1348. https://doi.org/10.3390/plants13101348
Ku S, Park G, Jang YP. Two-Dimensional High-Performance Thin-Layer Chromatography with Bioautography for Distinguishing Angelicae Dahuricae Radix Varieties: Chemical Fingerprinting and Antioxidant Profiling. Plants. 2024; 13(10):1348. https://doi.org/10.3390/plants13101348
Chicago/Turabian StyleKu, Sejin, Geonha Park, and Young Pyo Jang. 2024. "Two-Dimensional High-Performance Thin-Layer Chromatography with Bioautography for Distinguishing Angelicae Dahuricae Radix Varieties: Chemical Fingerprinting and Antioxidant Profiling" Plants 13, no. 10: 1348. https://doi.org/10.3390/plants13101348






