Cell-Autonomous and Non-Cell-Autonomous Mechanisms Concomitantly Regulate the Early Developmental Pattern in the Kelp Saccharina latissima Embryo
Abstract
:1. Introduction
2. Results
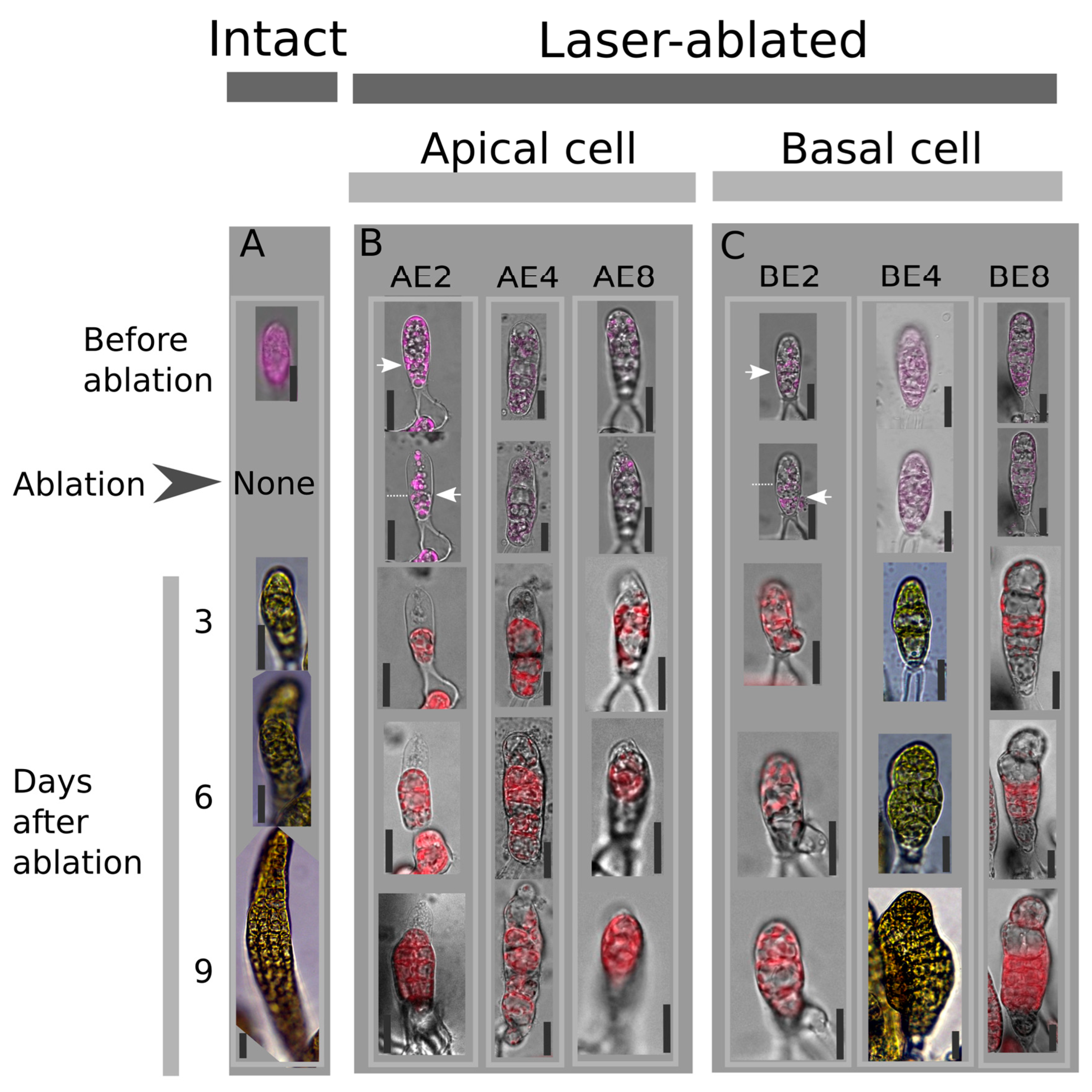
2.1. Ablation of the Apical Cell Is Not Offset by Faster Growth
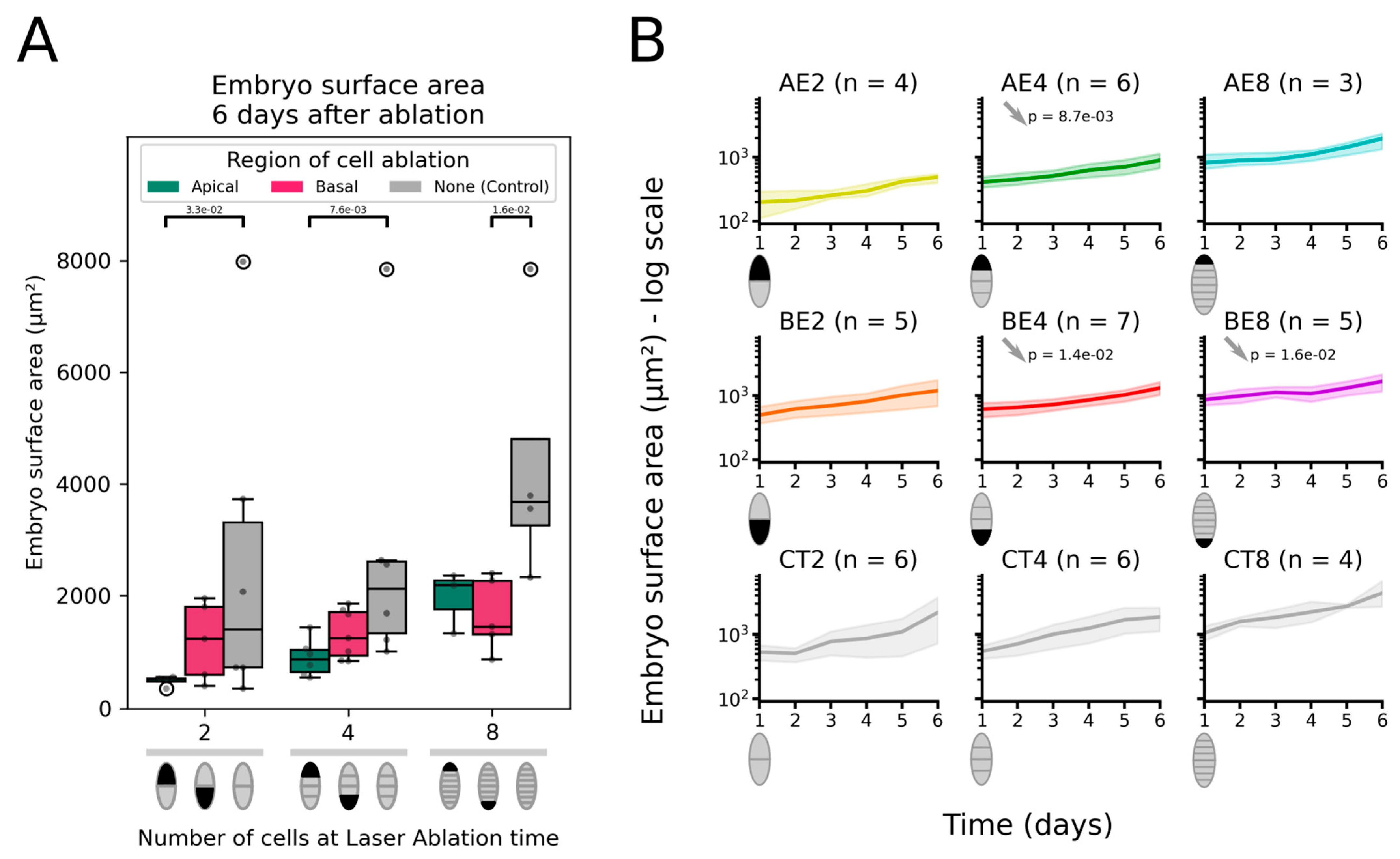
2.1.1. Surface Area
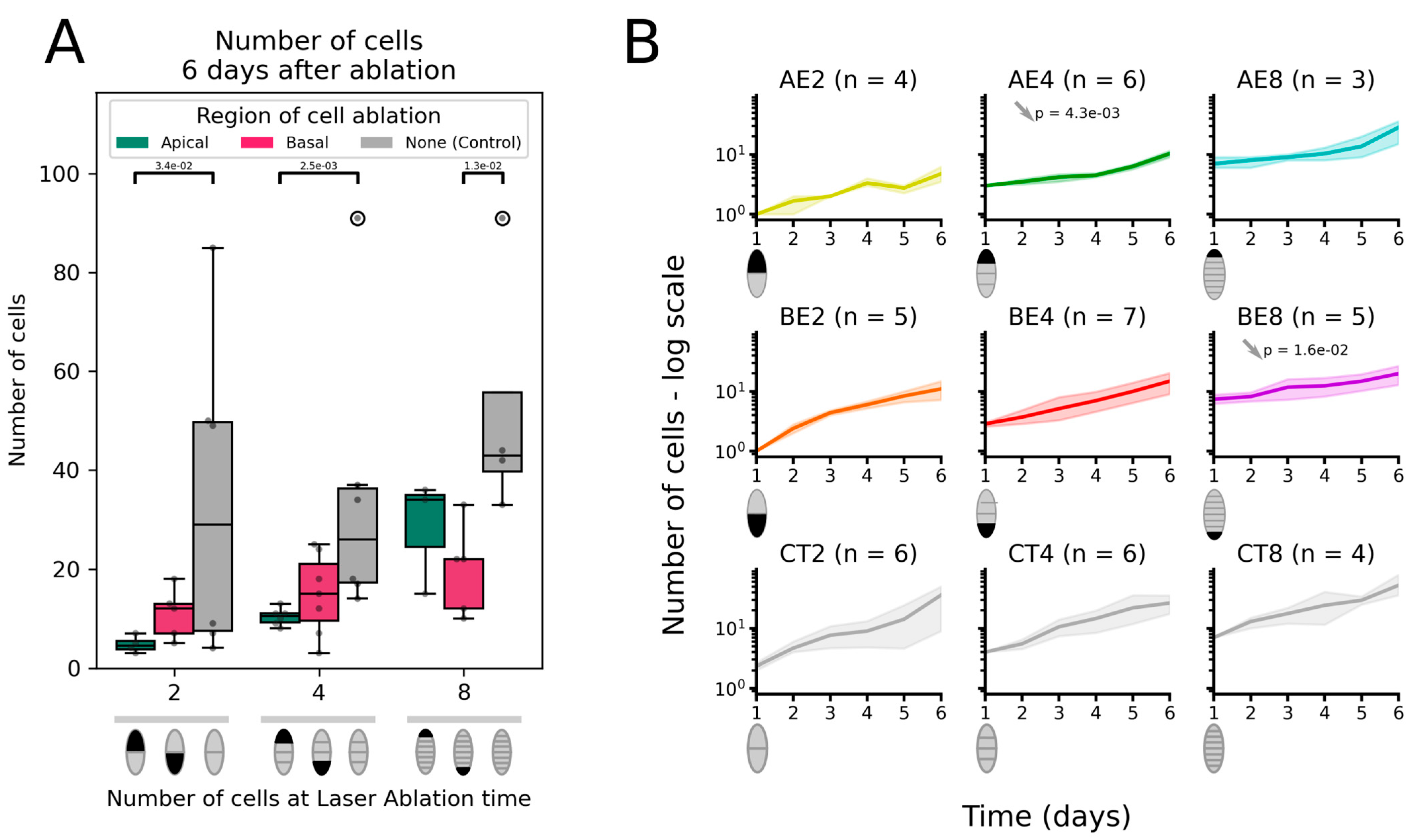
2.1.2. Number of Cells
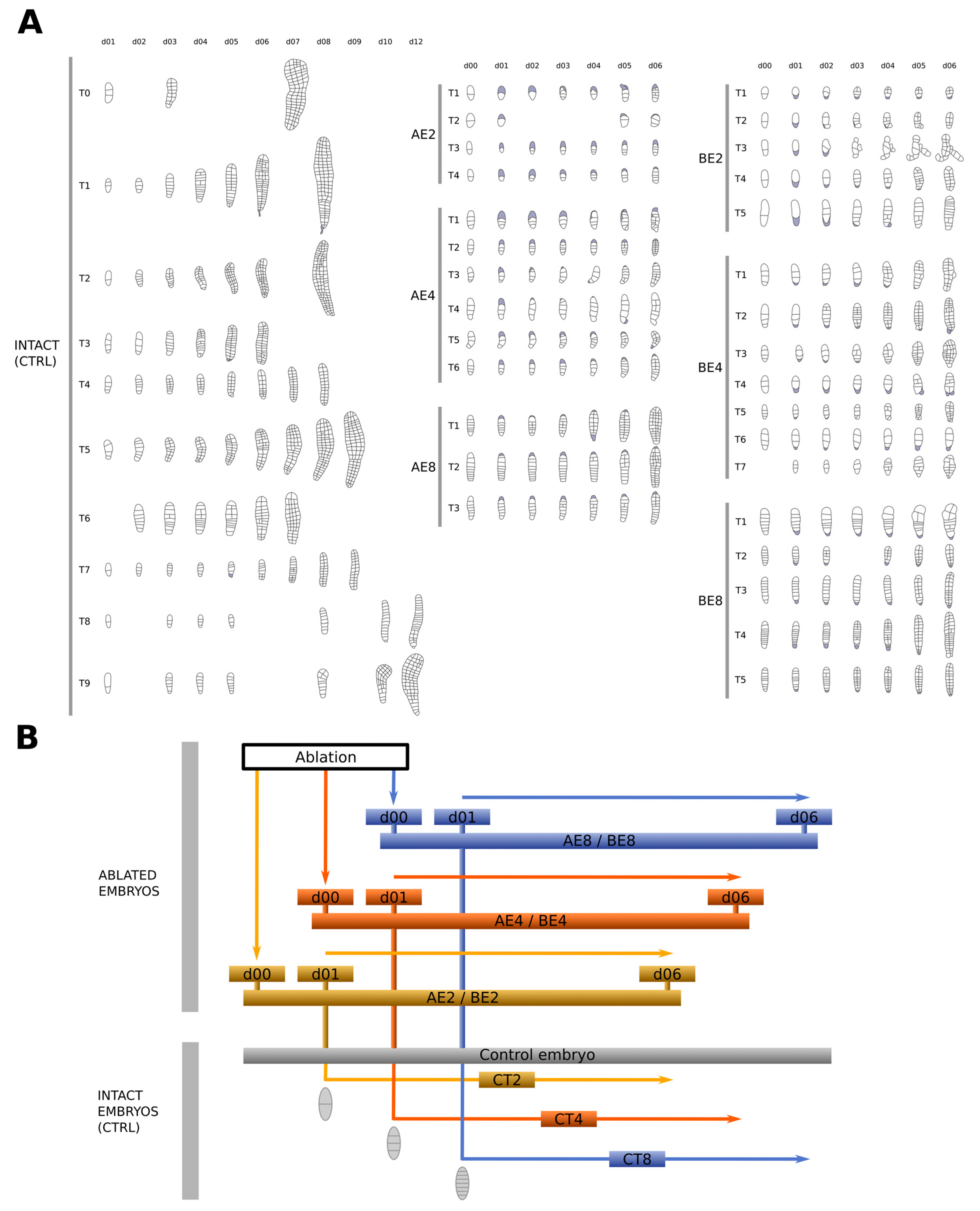
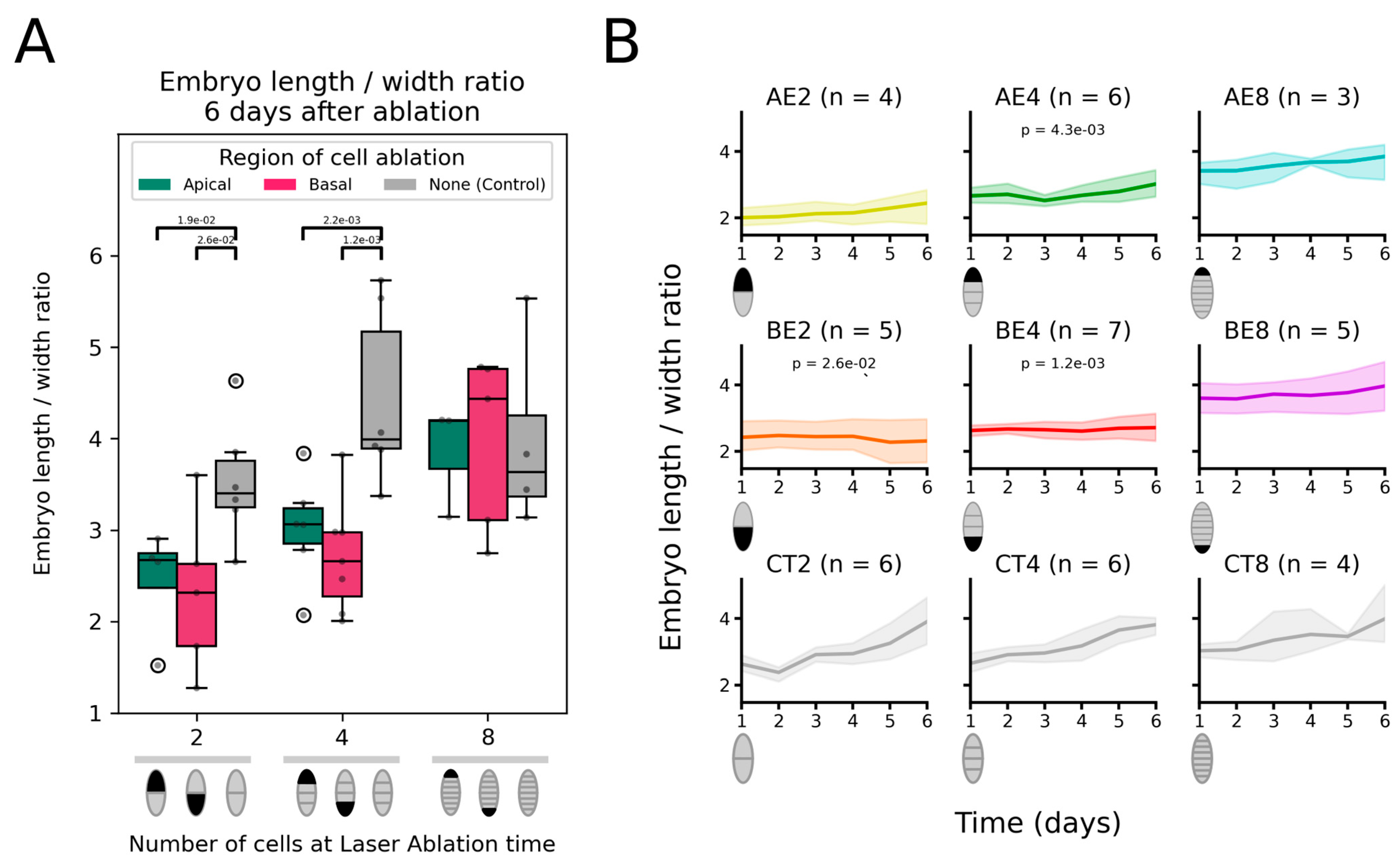
2.2. Ablated Embryos Cannot Recover the Loss of Their Initial Longitudinal Axis
2.3. Basal Cells Negatively Control Longitudinal Cell Division
3. Discussion
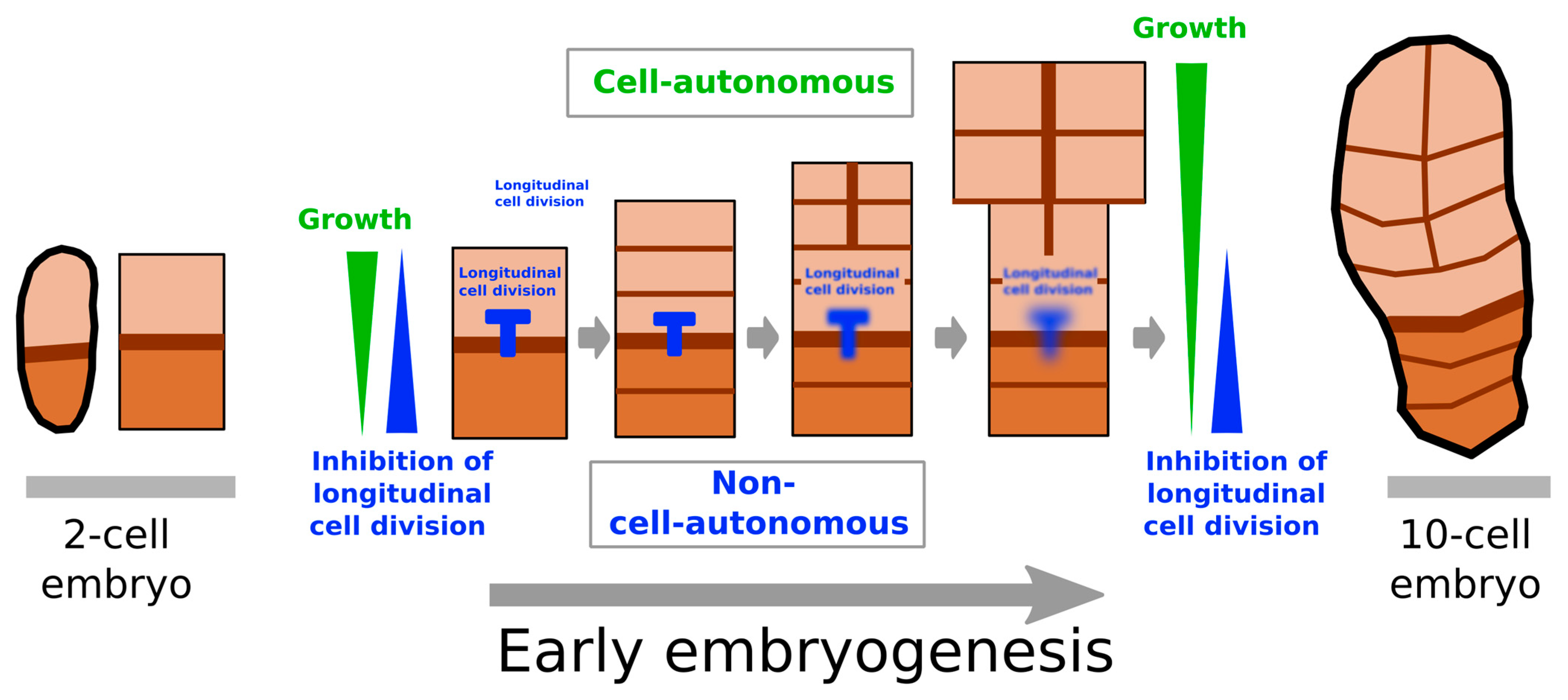
3.1. Concomitant Cell-Autonomous and Non-Cell-Autonomous Controls of Growth
3.2. The Impact of Ablation Is Greatest before the Four-Cell Stage
4. Materials and Methods
4.1. Algal Culture
4.2. Laser Irradiation of Embryo Cells at Different Developmental Stage
4.3. Image Acquisition
4.4. Manual Segmentation
4.5. Quantitative Morphometry
4.6. Statistical Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fritsch, F.E. The Structure and Reproduction of the Algae, Volume II; Foreword, Phaeophyceae, Rhodophyceae, Myxophyceae; Cambridge University Press: Cambridge, UK, 1965. [Google Scholar]
- Bogaert, K.A.; Beeckman, T.; De Clerck, O. Two-Step Cell Polarization in Algal Zygotes. Nat. Plants 2017, 3, 16221. [Google Scholar] [CrossRef] [PubMed]
- Motomura, T. Ultrastructure of Fertilization in Laminaria Angustata (Phaeophyta, Laminariales) with Emphasis on the Behavior of Centrioles, Mitochondria and Chloroplasts of the Sperm. J. Phycol. 1990, 26, 80–89. [Google Scholar] [CrossRef]
- Berger, F.; Brownlee, C. Establishment of the Apical-Basal Axis in Multicellular Plant Embryos. Biol. Cell 1995, 84, 7–11. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Beeckman, T.; De Clerck, O. Photopolarization of Fucus Zygotes Is Determined by Time Sensitive Vectorial Addition of Environmental Cues during Axis Amplification. Front. Plant Sci. 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Basu, S.; Brady, S.R.; Luciano, R.L.; Muday, G.K. Interactions between Auxin Transport and the Actin Cytoskeleton in Developmental Polarity of Fucus Distichus Embryos in Response to Light and Gravity. Plant Physiol. 2004, 135, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Goddard, H.; Manison, N.F.; Tomos, D.; Brownlee, C. Elemental Propagation of Calcium Signals in Response-Specific Patterns Determined by Environmental Stimulus Strength. Proc. Natl. Acad. Sci. USA 2000, 97, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; Taylor, A.R.; Ryan, K.P.; Sousa-Pinto, I.; Brown, M.T.; Brownlee, C. Spatiotemporal Patterning of Reactive Oxygen Production and Ca2+ Wave Propagation in Fucus Rhizoid Cells. Plant Cell 2002, 14, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Corellou, F.; Potin, P.; Brownlee, C.; Kloareg, B.; Bouget, F.-Y. Inhibition of the Establishment of Zygotic Polarity by Protein Tyrosine Kinase Inhibitors Leads to an Alteration of Embryo Pattern in Fucus. Dev. Biol. 2000, 219, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Taylor, A.; Brownlee, C. Cell Fate Determination by the Cell Wall in Early Fucus Development. Science 1994, 263, 1421–1423. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Zakka, E.E.; Coelho, S.M.; De Clerck, O. Polarization of Brown Algal Zygotes. Semin. Cell Dev. Biol. 2023, 134, 90–102. [Google Scholar] [CrossRef]
- Hable, W.E.; Miller, N.R.; Kropf, D.L. Polarity Establishment Requires Dynamic Actin in Fucoid Zygotes. Protoplasma 2003, 221, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Alessa, L.; Kropf, D.L. F-Actin Marks the Rhizoid Pole in Living Pelvetia Compressa Zygotes. Development 1999, 126, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lüning, K. Egg Release in Gametophytes of Laminaria Saccharina: Induction by Darkness and Inhibition by Blue Light and u.v. Br. Phycol. J. 1981, 16, 379–393. [Google Scholar] [CrossRef]
- Sauvageau, C. Recherches Sur Les Laminaires Des Cotes de France. Mem. Acad. Sci. 1918, 56, 1–240. [Google Scholar]
- Theodorou, I.; Charrier, B. The Shift to 3D Growth during Embryogenesis of Kelp Species, Atlas of Cell Division and Differentiation of Saccharina Latissima. Development 2023, 150, dev201519. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.D.; Liu, A.; Du, A.; Bhirgoo, P.D.; Fang-Yen, C. Thermal Laser Ablation with Tunable Lesion Size Reveals Multiple Origins of Seizure-like Convulsions in Caenorhabditis Elegans. Sci. Rep. 2021, 11, 5084. [Google Scholar] [CrossRef] [PubMed]
- Angelo, J.R.; Tremblay, K.D. Laser-Mediated Cell Ablation during Post-Implantation Mouse Development. Dev. Dyn. 2013, 242, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.B.; Hammarlund, M. Axon Regeneration in C. Elegans: Worming Our Way to Mechanisms of Axon Regeneration. Exp. Neurol. 2017, 287, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.S.; Holzemer, N.F.; Wingert, R.A. Laser Ablation of the Zebrafish Pronephros to Study Renal Epithelial Regeneration. J. Vis. Exp. 2011, 54, 2845. [Google Scholar] [CrossRef]
- Reinhardt, D. Microsurgical and Laser Ablation Analysis of Interactions between the Zones and Layers of the Tomato Shoot Apical Meristem. Development 2003, 130, 4073–4083. [Google Scholar] [CrossRef]
- van den Berg, C.; Hage, W.; Weisbeek, P.; Scheres, B. Laser Ablation In Arabidopsis Roots: A Tool To Study Cell-To-Cell Communication. In Proceedings of the Cellular Integration of Signalling Pathways in Plant Development; Lo Schiavo, F., Last, R.L., Morelli, G., Raikhel, N.V., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 237–250. [Google Scholar]
- Bouget, F.Y.; Berger, F.; Brownlee, C. Position Dependent Control of Cell Fate in the Fucus Embryo: Role of Intercellular Communication. Development 1998, 125, 1999–2008. [Google Scholar]
- Boscq, S.; Dutertre, S.; Theodorou, I.; Charrier, B. Targeted Laser Ablation in the Embryo of Saccharina Latissima. J. Vis. Exp. JoVE 2022, e63518. [Google Scholar] [CrossRef] [PubMed]
- Nagasato, C.; Terauchi, M.; Tanaka, A.; Motomura, T. Development and Function of Plasmodesmata in Zygotes of Fucus Distichus. Bot. Mar. 2015, 58, 229–238. [Google Scholar] [CrossRef]
- Terauchi, M.; Nagasato, C.; Motomura, T. Plasmodesmata of Brown Algae. J. Plant Res. 2015, 128, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, A.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Le Panse, S.; Stewart, S.; Scornet, D.; Cock, J.M.; Ljung, K.; et al. Auxin Metabolism and Function in the Multicellular Brown Alga Ectocarpus Siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Gaillochet, C.; Lohmann, J.U. The Never-Ending Story: From Pluripotency to Plant Developmental Plasticity. Development 2015, 142, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.M.; Nichols, J.; Schröter, C. A Molecular Basis for Developmental Plasticity in Early Mammalian Embryos. Development 2013, 140, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Boscq, S.; Billoud, B.; Theodorou, I.; Joemmanbaks, T.; Charrier, B. MUM, a maternal unknown message, inhibits early establishment of the medio-lateral axis in the embryo of the kelp Saccharina latissima. bioRxiv 2024. [Google Scholar] [CrossRef]
- Rusig, A.-M.; Ouichou, A.; Le Guyader, H.; Ducreux, G. Ontogenesis in the Fucophyceae: Case Studies and Comparison of Fucoid Zygotes and Sphacelaria Apical Cells. Cryptogam. Algol. 2001, 22, 227–248. [Google Scholar] [CrossRef]
- Le Bail, A.; Billoud, B.; Le Panse, S.; Chenivesse, S.; Charrier, B. ETOILE Regulates Developmental Patterning in the Filamentous Brown Alga Ectocarpus Siliculosus. Plant Cell 2011, 23, 1666–1678. [Google Scholar] [CrossRef]
- Theodorou, I.; Charrier, B. Brown Algae: Ectocarpus and Saccharina as Experimental Models for Developmental Biology. In Handbook of Marine Model Organisms in Experimental Biology; Boutet, A., Schierwater, B., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 27–47. ISBN 978-1-00-321750-3. [Google Scholar]
- Theodorou, I.; Opsahl-Sorteberg, H.-G.; Charrier, B. Preparation of Zygotes and Embryos of the Kelp Saccharina Latissima for Cell Biology Approaches. Bio-Protocol 2021, 11, e4132. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F.L. The Python Language Reference, Release 3.11.5, Python Software Foundation; Network Theory Ltd.: Godalming, UK, 2023; ISBN 978-1-906966-14-0. [Google Scholar]
- Rosin, P.L. Computing Global Shape Measures. In Handbook of Pattern Recognition and Computer Vision; World Scientific: Singapore, 2005; pp. 177–196. ISBN 978-981-256-105-3. [Google Scholar]
- Fletcher, A.G.; Osborne, J.M.; Maini, P.K.; Gavaghan, D.J. Implementing Vertex Dynamics Models of Cell Populations in Biology within a Consistent Computational Framework. Prog. Biophys. Mol. Biol. 2013, 113, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.D. Evolution of the Mechanisms That Establish the Embryonic Axes. Curr. Opin. Genet. Dev. 2006, 16, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.A.; Levine, M. Mosaic and Regulative Development: Two Faces of One Coin. Curr. Biol. 2006, 16, R236–R239. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscq, S.; Billoud, B.; Charrier, B. Cell-Autonomous and Non-Cell-Autonomous Mechanisms Concomitantly Regulate the Early Developmental Pattern in the Kelp Saccharina latissima Embryo. Plants 2024, 13, 1341. https://doi.org/10.3390/plants13101341
Boscq S, Billoud B, Charrier B. Cell-Autonomous and Non-Cell-Autonomous Mechanisms Concomitantly Regulate the Early Developmental Pattern in the Kelp Saccharina latissima Embryo. Plants. 2024; 13(10):1341. https://doi.org/10.3390/plants13101341
Chicago/Turabian StyleBoscq, Samuel, Bernard Billoud, and Bénédicte Charrier. 2024. "Cell-Autonomous and Non-Cell-Autonomous Mechanisms Concomitantly Regulate the Early Developmental Pattern in the Kelp Saccharina latissima Embryo" Plants 13, no. 10: 1341. https://doi.org/10.3390/plants13101341




