Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Compounds (TPC) and Phenolic Acids
2.2. Flavonoids and Hydroxycinnamic Acids
2.3. Fatty Acids
3. Discussion
3.1. Total Phenolic Compounds (TPC)
3.2. Phenolic Acids
3.3. Flavonoids and Hydroxycinnamic Acids
3.4. Fatty Acids
3.5. Nutritional Importance and Future Implications
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Determination of Humidity
4.2.2. Preparation of Methanol Extract
4.2.3. Total Phenolic Compounds (TPC)
4.2.4. Gas Chromatography Coupled with Mass Spectrometry (GC-MS) for Phenolic and Fatty Acid Analysis
4.2.5. High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry (HPLC-MS/ESI) for Flavonoid and Hydroxycinnamic Acid Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De la Fuente, V.; Oggerin, M.; Rufo, L.; Rodríguez, N.; Ortuñez, E.; Sánchez-Mata, D.; Amils, R. A micromorphological and phylogenetic study of Sarcocornia AJ Scott (Chenopodiaceae) on the Iberian Peninsula. Plant Biosyst. 2013, 147, 158–173. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, V.; Rufo, L.; Rodríguez, N.; Sánchez-Mata, D.; Franco, A.; Amils, R. A study of Sarcocornia AJ Scott (Chenopodiaceae) from Western Mediterranean Europe. Plant Biosyst. 2016, 150, 343–356. [Google Scholar] [CrossRef]
- De la Fuente, V.; Sánchez-Gavilán, I.; Ramírez, E.; Rufo, L.; Sánchez-Mata, D. Morphological Variability of Halophytes: Salicornioideae on Iberian Peninsula. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Rufo, L.; De La Fuente, V.; Sánchez-Mata, D. Sarcocornia plant communities of the Iberian Peninsula and the Balearic Islands. Phytocoenologia 2016, 46, 383–396. [Google Scholar] [CrossRef]
- Rufo, L.; Iglesias-López, M.T.; De La Fuente, V. The endemic halophyte Sarcocornia carinata Fuente, Rufo & Sánchez-Mata (Chenopodiaceae) in relation to environmental variables: Elemental composition and biominerals. Plant Soil 2021, 460, 189–209. [Google Scholar]
- Ramírez, E.; Rufo, L.; Sánchez-Mata, D.; De La Fuente, V. Arthrocaulon meridionalis (Chenopodiaceae), a new species of Mediterranean flora. Mediterr. Bot. 2019, 40, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Alhdad, G.M.; Seal, C.E.; Al-Azzawi, M.J.; Flowers, T.J. The effect of combined salinity and waterlogging on the halophyte Suaeda maritima: The role of antioxidants. Environ. Experiment. Bot. 2013, 87, 120–125. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Analysis 2017, 59, 35–42. [Google Scholar] [CrossRef]
- De la Fuente, V.; Rufo, L.; Sánchez-Gavilán, I.; Ramírez, E.; Rodríguez, N.; Amils, R. Plant tissues and embryos biominerals in Sarcocornia pruinosa, a halophyte from the Río Tinto salt marshes. Minerals 2018, 8, 505. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Salicornia patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef]
- Barcia-Piedras, J.M.; Perez-Romero, J.A.; Mateos-Naranjo, E.; Camacho, M.; Redondo-Gómez, S. Effect of prior salt experience on desalination capacity of the halophyte Arthrocnemum macrostachyum. Desalination 2019, 463, 50–54. [Google Scholar] [CrossRef]
- El-Naker, N.A.; Yousef, A.F.; Yousef, L.F. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem. Rev. 2020, 19, 1427–1448. [Google Scholar] [CrossRef]
- Riquelme, J.; Olaeta, J.A.; Galvez, L.; Undurraga, P.; Fuentealba, C.; Osses, A.; Orellana, J.; Gallardo, J.; Pedreschi, R. Nutritional and functional characterization of wild and cultivated Sarcocornia neii grown in Chile. Cien. Investig. Agr. 2016, 43, 283–293. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Scientia Horticult. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Experiment. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Singh, D.; Buhmann, A.K.; Flowers, T.J.; Seal, C.E.; Papenbrock, J. Salicornia as a crop plant in temperate regions: Selection of genetically characterized ecotypes and optimization of their cultivation conditions. AoB Plants 2014, 6, plu071. [Google Scholar] [CrossRef] [Green Version]
- Paraskevopoulou, A.; Mitsios, I.; Fragkakis, I.; Nektarios, P.; Ntoulas, N.; Londra, P.; Papafotiou, M. The growth of Arthrocnemum macrostachyum and Halimione portulacoides in an extensive green roof system under two watering regimes. Agricult. Agr. Sci. Proc. 2015, 4, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval- Jouve: From gathering of wild plants to some attempts of cultivation in Apulia region (southern Italy). Genet. Resour. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Rufo, L.; Rodríguez, N.; Amils, R.; de la Fuente, V.; Jiménez-Ballesta, R. Surface geochemistry of soils associated to the Tinto River (Huelva, Spain). Sci. Total Environ. 2007, 378, 223–227. [Google Scholar] [CrossRef]
- Rufo, L.; de la Fuente, V. Chemical and mineralogical characterization of the soils of the main plant communities of the “Río Tinto” Basin. Schironia 2010, 9, 5–13. [Google Scholar]
- Ramírez, E.; Rufo, L.; Sánchez-Mata, D.; Sánchez-Gavilán, I.; de la Fuente, V. Arthrocnemum macrostachyum plant communities in the Iberian Peninsula, Balearic and Canary Islands (Spain and Portugal). In Tools for Landscape-Scale Geobotany and Conservation; Springer: Cham, Switzerland, 2021; pp. 231–245. [Google Scholar]
- Sánchez-Gavilán, I.; Rufo, L.; Rodriguez, N.; de la Fuente, V. On the elemental composition of the Mediterranean euhalophyte Salicornia patula Duval-Jouve (Chenopodiaceae) from saline habitats in Spain (Huelva, Toledo and Zamora). Environ. Sci. Pollut. Res. 2021, 28, 2719–2727. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Bhaskar, S.D.; Yadav, K.; Penna, S. Halophytes: Prospective Plants for Future. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Phenolic acids from plants: Extraction and application to human health. Stud. Nat. Prod. Chem. 2018, 58, 389–417. [Google Scholar]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Varela, J.; Barreira, L. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef] [PubMed]
- Barroca, M.J.; Guiné, R.P.; Amado, A.M.; Ressurreição, S.; da Silva, A.M.; Marques, M.P.M.; de Carvalho, L.B. The drying process of Sarcocornia perennis: Impact on nutritional and physico-chemical properties. J. Food Sci. Tech. 2020, 57, 4443–4458. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima. J. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ge, S.; Li, S.; Lin, H.; Lin, S. Anti-obesity effect of transcinnamic acid on HepG2 cells and HFD-fed mice. Food Chem. Toxicol. 2020, 137, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Bertin, R.L.; Gonzaga, L.V.; Borges, G.D.S.C.; Azevedo, M.S.; Maltez, H.F.; Heller, M.; Fett, R. Nutrient composition and, identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC–ESI-MS/MS. Food Res. Int. 2013, 55, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.S.B.; Chaves, F.C.; Rombaldi, C.V.; Souza, C.R. Bioactive compounds and antioxidant activity of three biotypes of the sea asparagus Sarcocornia ambigua (Michx.) MA Alonso & MB Crespo: A halophytic crop for cultivation with shrimp farm effluent. S. Afr. J. Bot. 2018, 117, 95–100. [Google Scholar]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; uz Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pakistan J. Agricult. Res. 2019, 32, 8. [Google Scholar] [CrossRef]
- Panth, N.; Park, S.-H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Feng, Z.T.; Yuan, F.; Guo, J.R.; Suo, S.S.; Wang, B.S. Identification and functional analysis of the autofluorescent substance in Limonium bicolor salt glands. Plant Physiol. 2015, 97, 20–27. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Anatomical adaptations of halophytes. In A Review of Classic Literature and Recent Findings; Springer International Publishing: Cham, Switzerland, 2017; p. 338. [Google Scholar]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.N.; Oprica, L. Halophytes as possible source of antioxidant compounds, in a scenario based on threatened agriculture and food crisis. Iran. J. Public Health 2015, 44, 11–53. [Google Scholar]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversias. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Saura-Calixto, F. Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four European countries. Food Res. Int. 2015, 74, 315–323. [Google Scholar] [CrossRef]
- Buhmann, A.; Papenbrock, J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 2013, 40, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, D.; Li, M.; Shi, L. Metabolic Profiles Reveal Changes in Wild and Cultivated Soybean Seedling Leaves under Salt Stress. PLoS ONE 2016, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’oca, M.G.; Morón-Villarreyes, J.A.; Lemões, J.S.; Costa, C.S. Fatty acids composition in seeds of the South American glasswort Sarcocornia ambigua. Anais Acad. Brasil. Ciên. 2012, 84, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maatallah Zaier, M.; Ciudad-Mulero, M.; Cámara, M.; Pereira, C.; Ferreira, I.; Achour, L.; Kacem, A. Revalorization of Tunisian wild Amaranthaceae halophytes: Nutritional composition variation in two different phenotypes stages. J. Food Comp. Anal. 2020, 89, 103463. [Google Scholar] [CrossRef]
- Duarte, B.; Caçador, I.; Matos, A.R. Lipid landscape remodelling in Sarcocornia fruticosa green and red physiotypes. Plant Physiol. Biochem. 2020, 157, 128–137. [Google Scholar] [CrossRef]
- Custódio, L.; Ferreira, A.C.; Pereira, H.; Silvestre, L.; Vizetto-Duarte, C.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. The marine halophytes Carpobrotus edulis L. and Arthrocnemum macrostachyum L. are potential sources of nutritionally important PUFAs and metabolites with antioxidant, metal chelating and anticholinesterase inhibitory activities. Bot. Mar. 2012, 55, 281–288. [Google Scholar] [CrossRef]
- Cámara, M.; Fernández-Ruiz, V.; Ruiz-Rodríguez, B.M. Wild Edible Plants as Sources of Carotenoids, Fibre, Phenolics and Other Non-Nutrient Bioactive Compounds. In Mediterranean Wild Edible Plants. Ethnobotany and Food Composition Tables; Sánchez-Mata, M.C., Tardío, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 187–205. [Google Scholar]
- Surówka, E.; Latowski, D.; Libik-Konieczny, M.; Miszalski, Z. 11 ROS Signalling, and Antioxidant Defence Network in Halophytes. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Boston, MA, USA, 2019; p. 179. [Google Scholar]
- Vizetto-Duarte, C.; Figueiredo, F.; Rodrigues, M.J.; Polo, C.; Rešek, E.; Custódio, L. Sustainable valorization of halophytes from the mediterranean area: A comprehensive evaluation of their fatty acid profile and implications for human and animal nutrition. Sustainability 2019, 11, 2197. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaparak, A.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia, L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef] [Green Version]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Andrés-Lacueva, C.; Medina-Remón, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventos, R.M. Phenolic compounds: Chemistry and occurrence in fruits and vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; De la Rosa, L., Alvarez-Parrilla, E., Gonzalez-Aguilar, G.A.E., Eds.; John Wiley and Sons: Cambridge, UK, 2010; pp. 53–88. [Google Scholar]

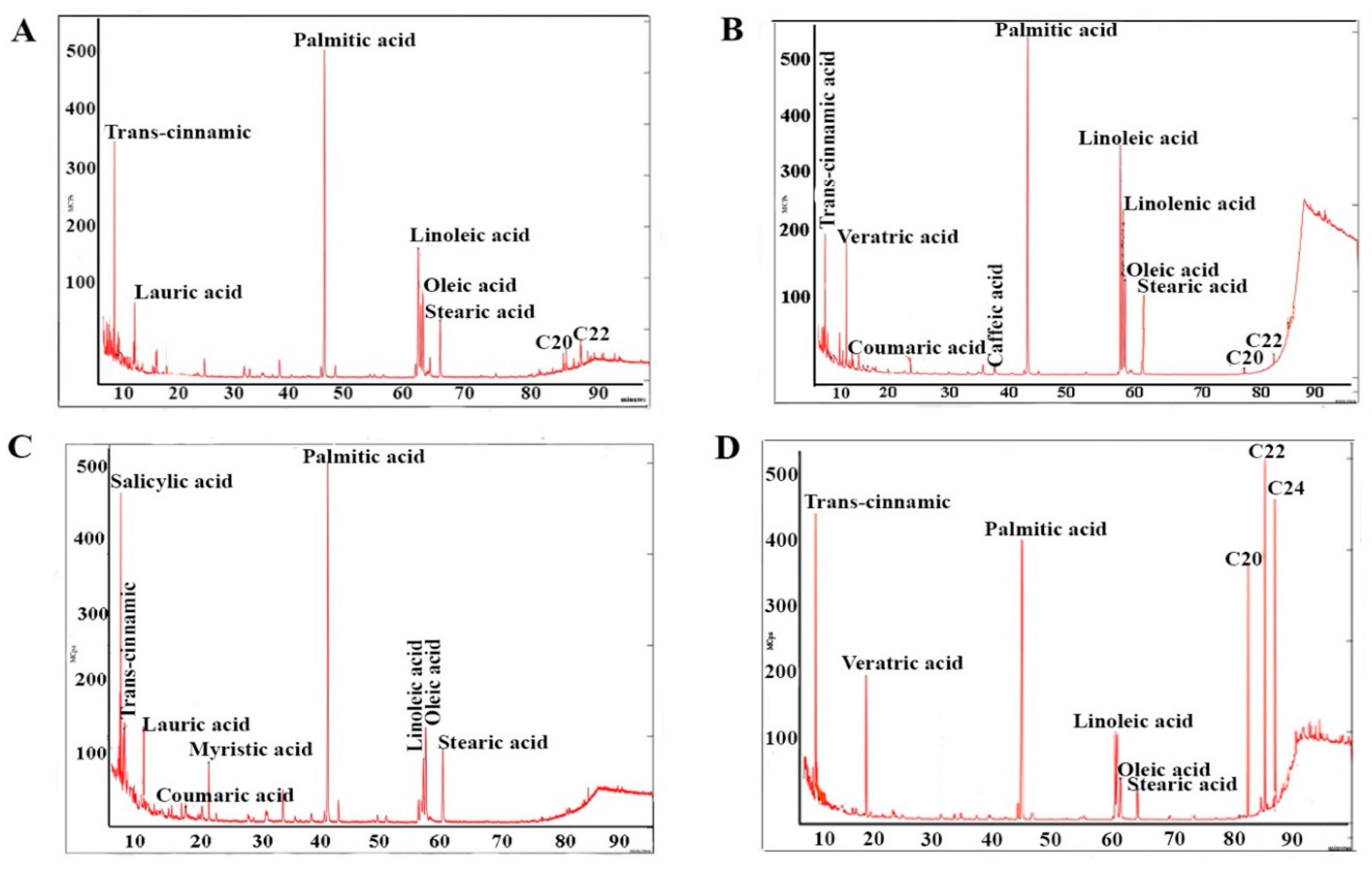
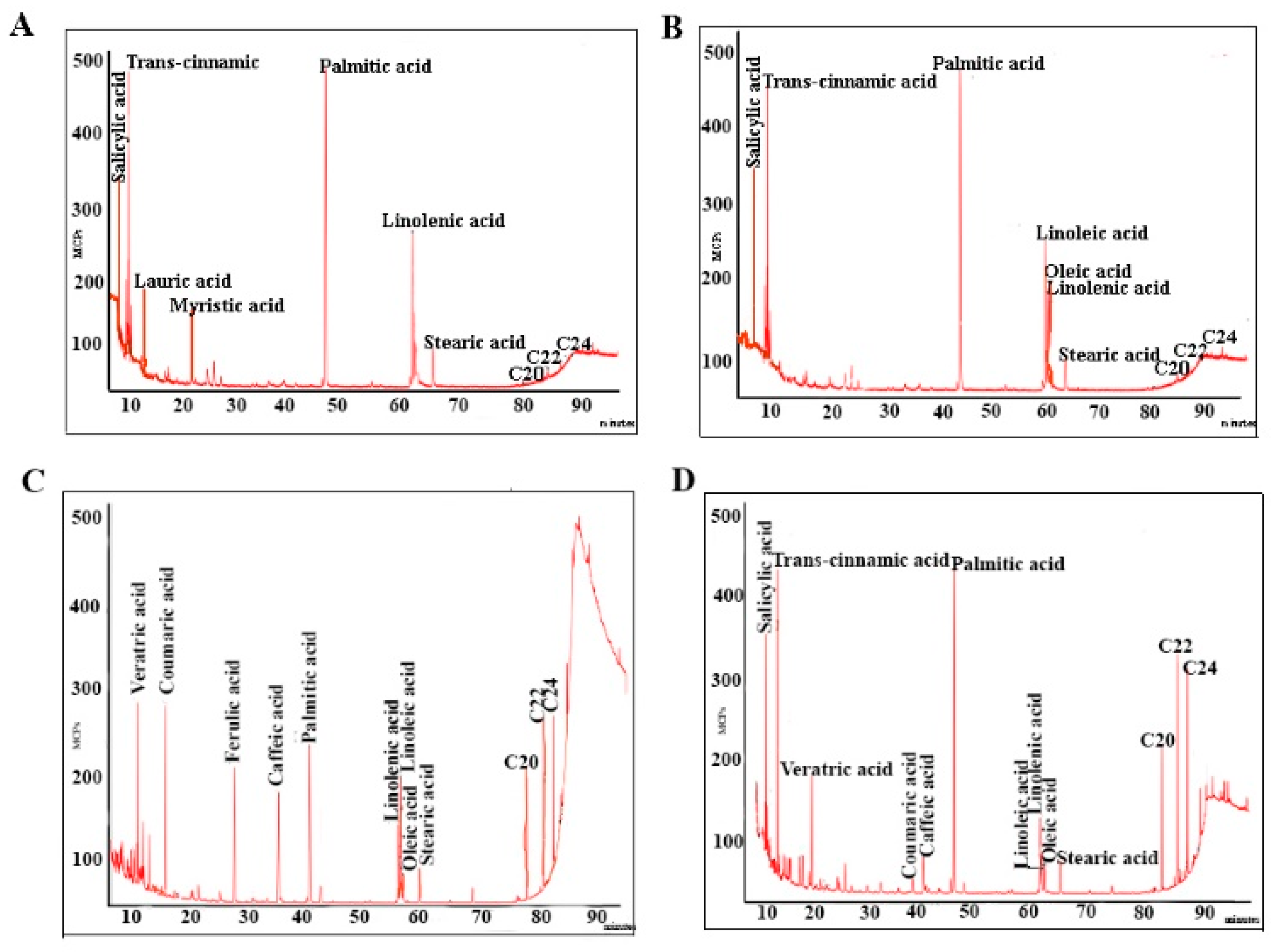
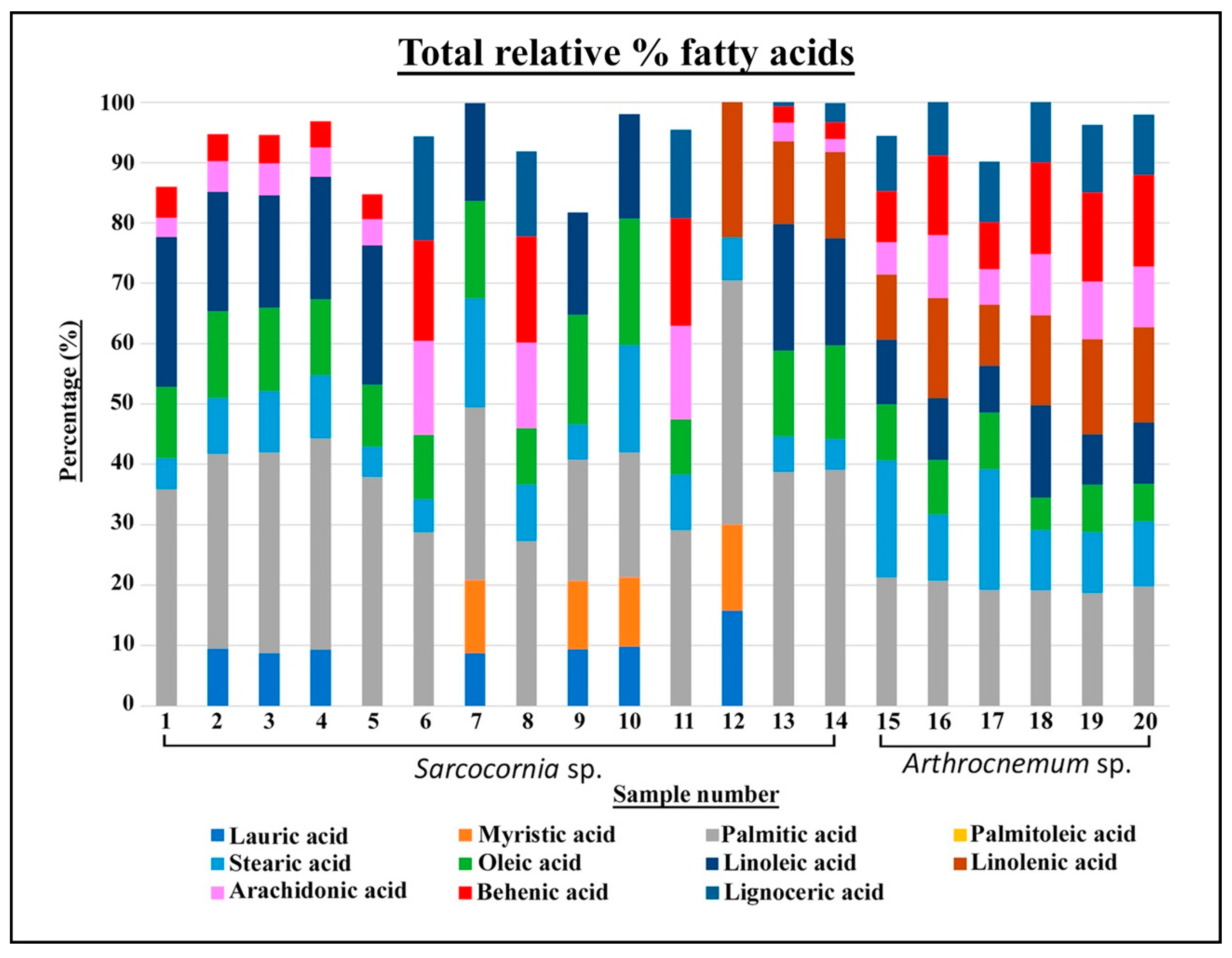
| ID | Sample | TPC ± SD | Humidity |
|---|---|---|---|
| 1 | S. alpini | 3.611 ± 0.107 | 10.23% |
| 2 | S. alpini | 3.569 ± 0.233 | 10.59% |
| 3 | S. alpini | 3.778 ± 0.231 | 11.80% |
| 4 | S. alpini | 3.480 ± 0.164 | 10.40% |
| 5 | S. alpini | 3.430 ± 0.093 | 11.30% |
| 6 | S. pruinosa | 3.892 ± 0.203 | 10.18% |
| 7 | S. pruinosa | 3.879 ± 0.207 | 10.53% |
| 8 | S. pruinosa | 3.404 ± 0.198 | 14.78% |
| 9 | S. pruinosa | 3.453 ± 0.064 | 14.44% |
| 10 | S. pruinosa | 3.299 ± 0.156 | 13.17% |
| 11 | S. pruinosa | 3.231 ± 0.089 | 13.65% |
| 12 | S. perennis | 3.407 ± 0.004 | 10.29% |
| 13 | S. perennis | 3.420 ± 0.139 | 13.16% |
| 14 | S. perennis | 3.460 ± 0.014 | 12.35% |
| 15 | A. macrostachyum | 4.680 ± 0.036 | 10.35% |
| 16 | A. macrostachyum | 4.891 ± 0.060 | 11.80% |
| 17 | A. macrostachyum | 4.803 ± 0.096 | 11.36% |
| 18 | A. macrostachyum | 4.220 ± 0.014 | 10.55% |
| 19 | A. macrostachyum | 4.850 ± 0.012 | 13.71% |
| 20 | A. macrostachyum | 4.770 ± 0.005 | 13.64% |
| Species | Flavonoid Compound | Experimental Mass M-H m/z | MS/MS (m/z) |
|---|---|---|---|
| S. alpini, S. pruinosa, S. perennis, A. macrostachyum | Luteolin | 287 | 285/290 |
| S. alpini | Cyanidin-3-O-arabinoside | 419 | 415/422 |
| Luteolin-7-glucoside | 448 | 446/450 | |
| S. pruinosa and A. macrostachyum | Dihydroquercetin | 304 | 303/310 |
| S. pruinosa | p-Coumaroyl tyrosine | 327 | 322/330 |
| A. macrostachyum | p-Coumaroyl-glucoside | 326 | 322/330 |
| ID | Sample | Geographic Location | Collection Date | MGRS Coordinates |
|---|---|---|---|---|
| 1 | S. alpini | Spain, Huelva, and San Juan del Puerto | 15 December 2017 | 29SPB9230 |
| 2 | S. alpini | Spain, Huelva, and La Rábida | 17 July 2018 | 29SPB8320 |
| 3 | S. alpini | Spain, Huelva, and Ayamonte | 18 July 2018 | 29SPB4122 |
| 4 | S. alpini | Portugal and Esteiro de Carrasqueira | 18 July 2018 | 29SPB3918 |
| 5 | S. alpini | Spain, Huelva, San Juan del Puerto, and saltmarshes of the “Embarcadero de Buitrón” | 7 August 2019 | 29SPB9031 |
| 6 | S. pruinosa | Spain, Huelva, and Tinto river estuary | 14 December 2017 | 29SPB8220 |
| 7 | S. pruinosa | Portugal, Tavira saltmarshes, and Santa Luzia | 18 July 2018 | 29SPB1906 |
| 8 | S. pruinosa | Spain, Huelva, and Punta del Moral | 18 July 2018 | 29SPB4717 |
| 9 | S. pruinosa | Spain, Huelva, and Odiel saltmarshes | 7 August 2019 | 29SPB7926 |
| 10 | S. pruinosa | Spain, Huelva, and La Rábida | 17 July 2018 | 29SPB8320 |
| 11 | S. pruinosa | Spain, Huelva, Odiel River, and “Cabeza Alta” | 14 December 2017 | 29SPB8024 |
| 12 | S. perennis | Portugal, Tavira saltmarshes, and Santa Luzia | 18 July 2018 | 29SPB1806 |
| 13 | S. perennis | Spain, Huelva and Tinto river estuary | 14 December 2017 | 29SPB8220 |
| 14 | S. perennis | Spain, Huelva and Punta del Moral, | 18 July 2018 | 29SPB4717 |
| 15 | A. macrostachyum | Spain, Zaragoza, and Belchite | 19 January 2017 | 30TXL8875 |
| 16 | A. macrostachyum | Spain, Madrid, and Colmenar de Oreja | 11 July 2018 | 31TVK5143 |
| 17 | A. macrostachyum | Spain, Huelva, and La Rábida | 15 December 2017 | 29SPB8320 |
| 18 | A. macrostachyum | Portugal, Marismas de Tavira, and Santa Luzia | 18 July 2018 | 29SPB1906 |
| 19 | A. macrostachyum | Spain, Huelva, San Juan del Puerto, and saltmarshes of the “Embarcadero de Buitrón” | 7 August 2019 | 29SPB9131 |
| 20 | A. macrostachyum | Spain, Huelva, and Ayamonte | 18 July 2018 | 29SPB4218 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gavilán, I.; Ramírez Chueca, E.; de la Fuente García, V. Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula. Plants 2021, 10, 2218. https://doi.org/10.3390/plants10102218
Sánchez-Gavilán I, Ramírez Chueca E, de la Fuente García V. Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula. Plants. 2021; 10(10):2218. https://doi.org/10.3390/plants10102218
Chicago/Turabian StyleSánchez-Gavilán, Irene, Esteban Ramírez Chueca, and Vicenta de la Fuente García. 2021. "Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula" Plants 10, no. 10: 2218. https://doi.org/10.3390/plants10102218
APA StyleSánchez-Gavilán, I., Ramírez Chueca, E., & de la Fuente García, V. (2021). Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula. Plants, 10(10), 2218. https://doi.org/10.3390/plants10102218






