Robotic Spot Spraying of Harrisia Cactus (Harrisia martinii) in Grazing Pastures of the Australian Rangelands
Abstract
:1. Introduction
1.1. Harrisia Cactus
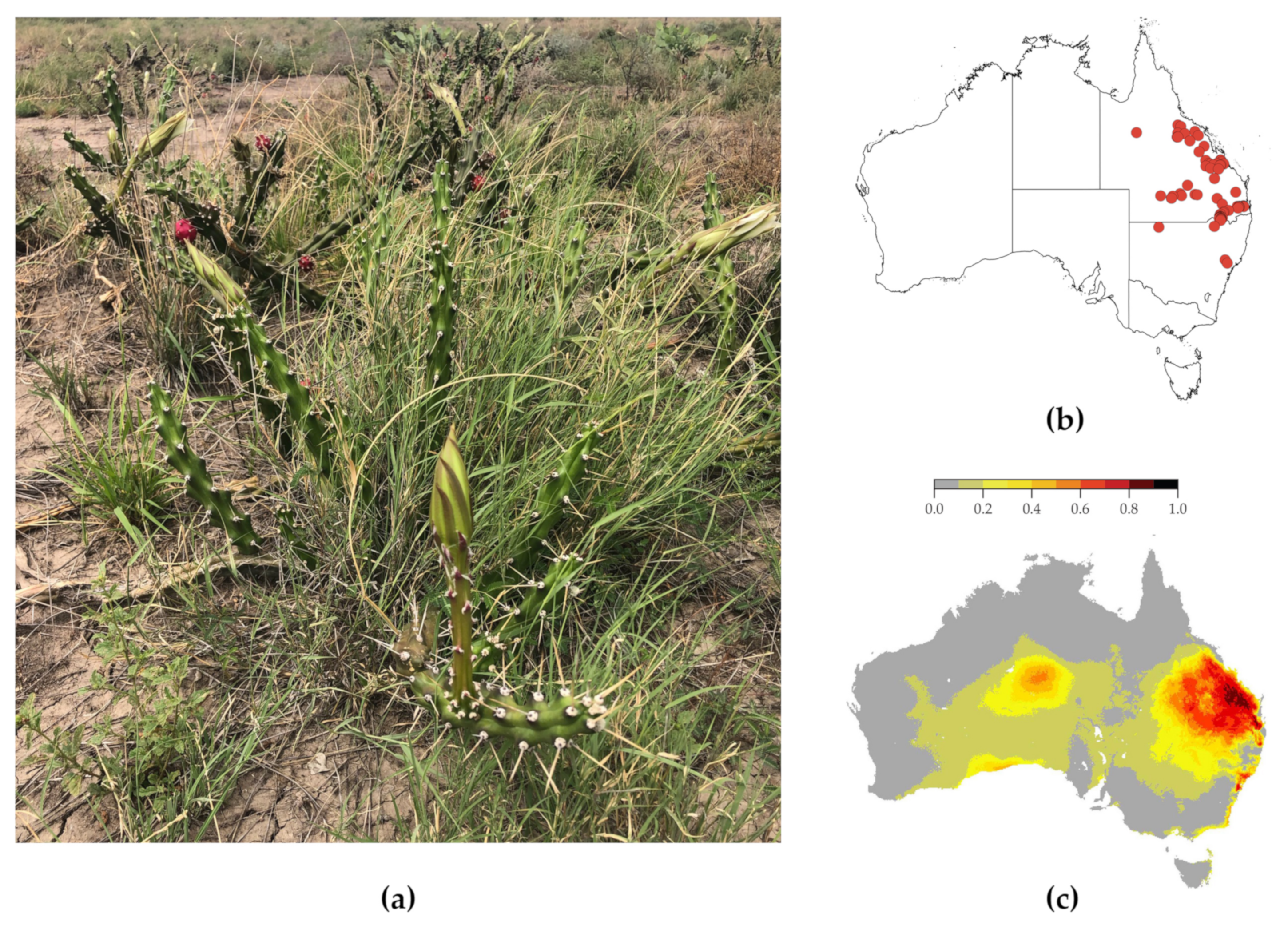
1.2. Control Methods
1.3. Robotic Spot Spraying
1.4. Overview
2. Materials and Methods
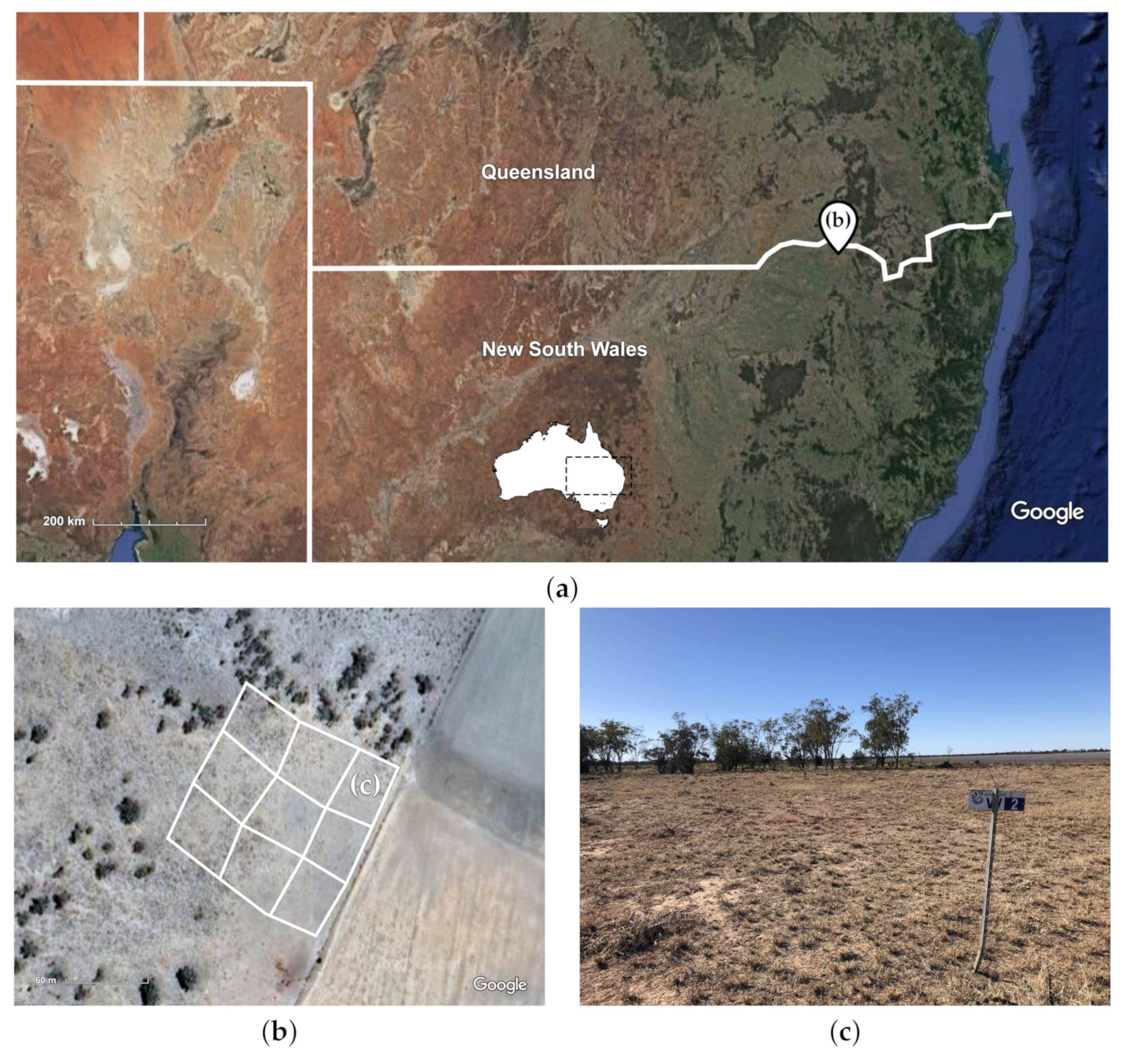
2.1. Trial Site
2.2. AutoWeed Detection Unit
2.3. Deep Learning
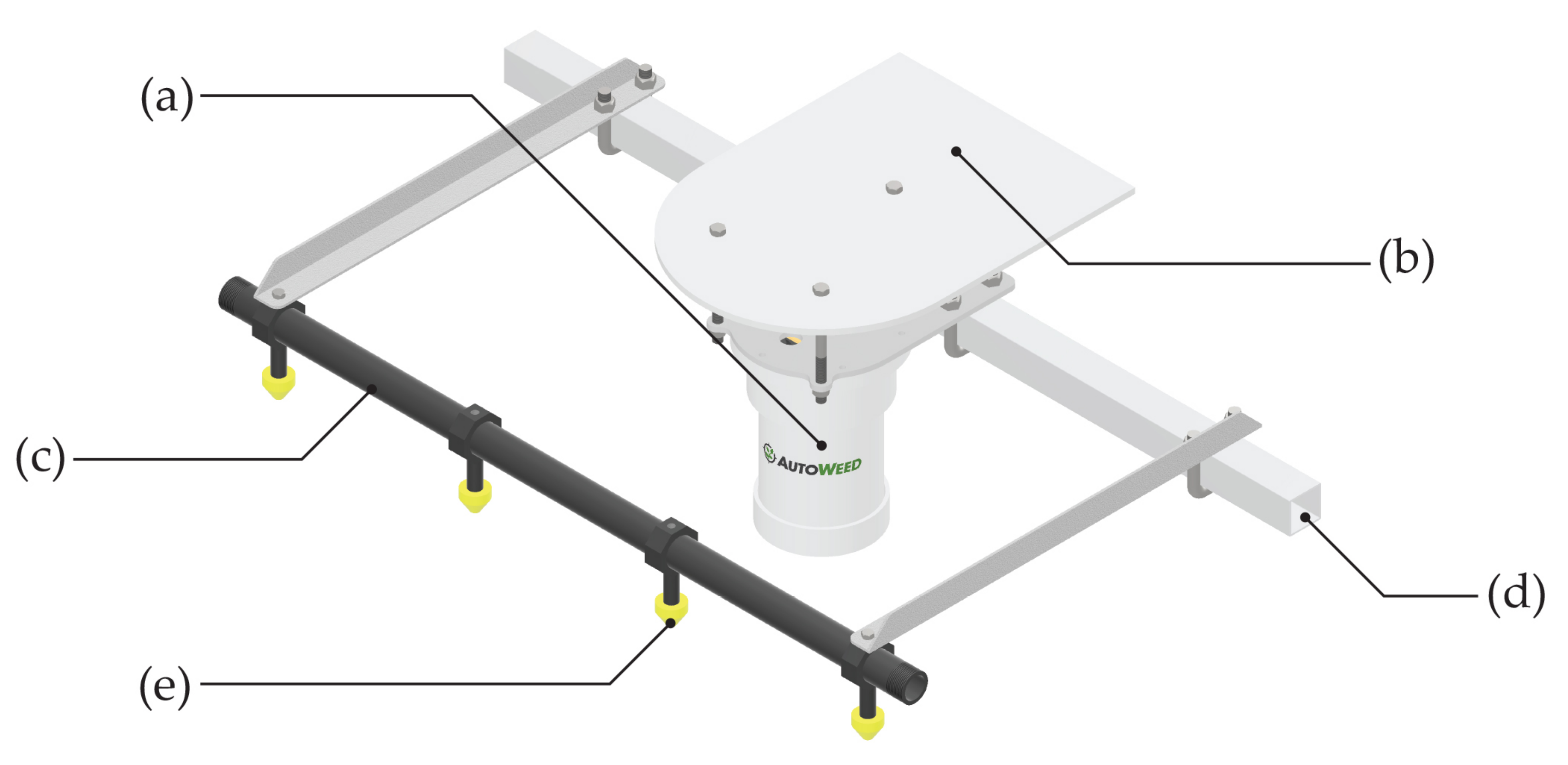
2.4. Vehicle and Spray Boom Design
2.5. Dataset Collection and Labelling
2.6. Chemical Mixture and Nozzle Selection
2.7. Experimental Methodology
- Low: 0 < n < 100
- Medium: 100 < n < 200
- High: n > 300
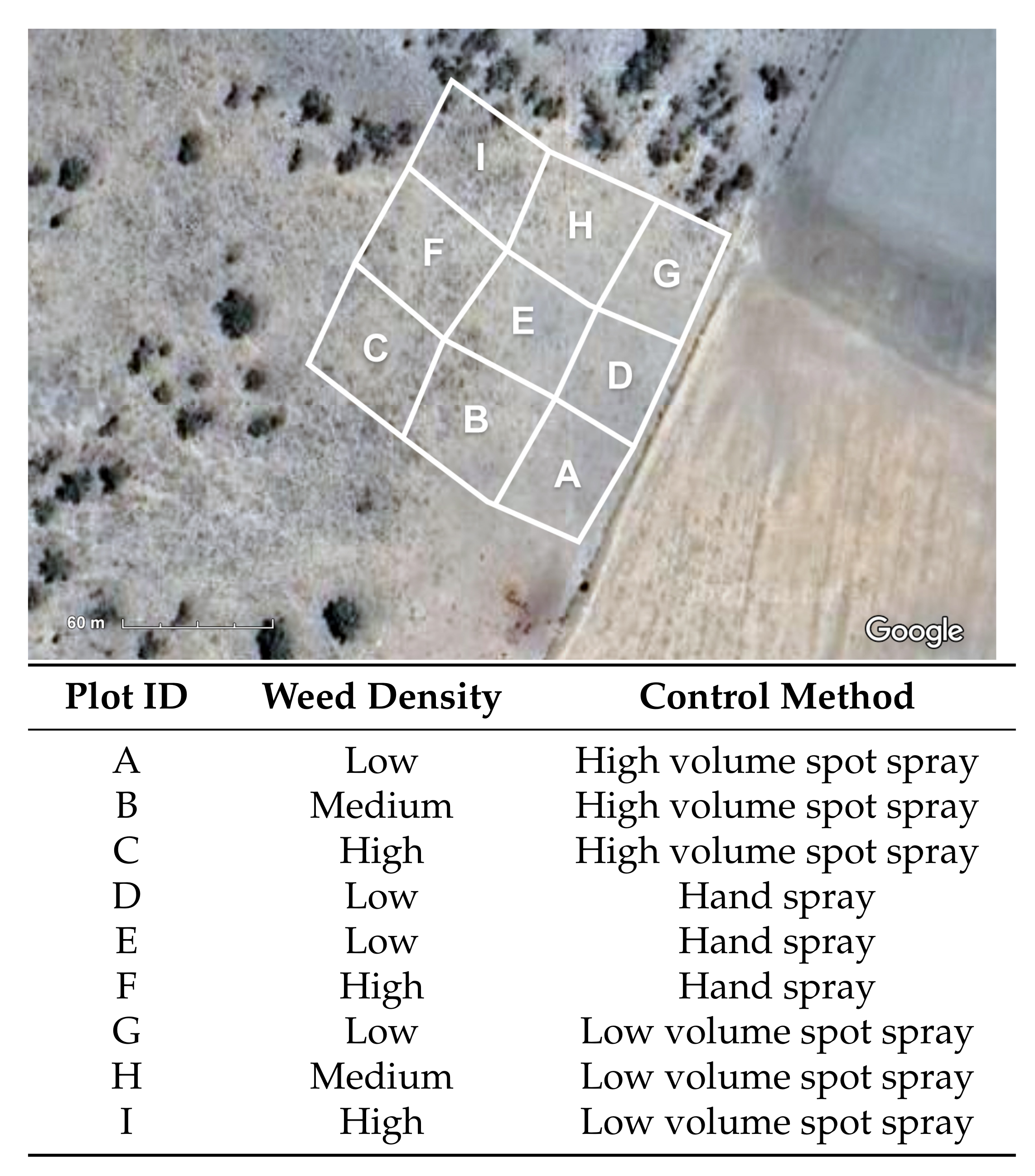
- Hand spray: Hand spraying with a handgun nozzle while traversing the plot with the UTV at varying speeds and manually identifying individual plants.
- Low volume spot spray: AutoWeed robotic spot spraying with a low volume (927 L/ha) application rate while traveling over the plot at <=10 km/hr in straight line transects.
- High volume spot spray: AutoWeed robotic spot spraying with a high volume (1547 L/ha) application rate while traveling over the plot at <=10 km/hr in straight line transects.
3. Results and Discussion
3.1. Dataset Collection
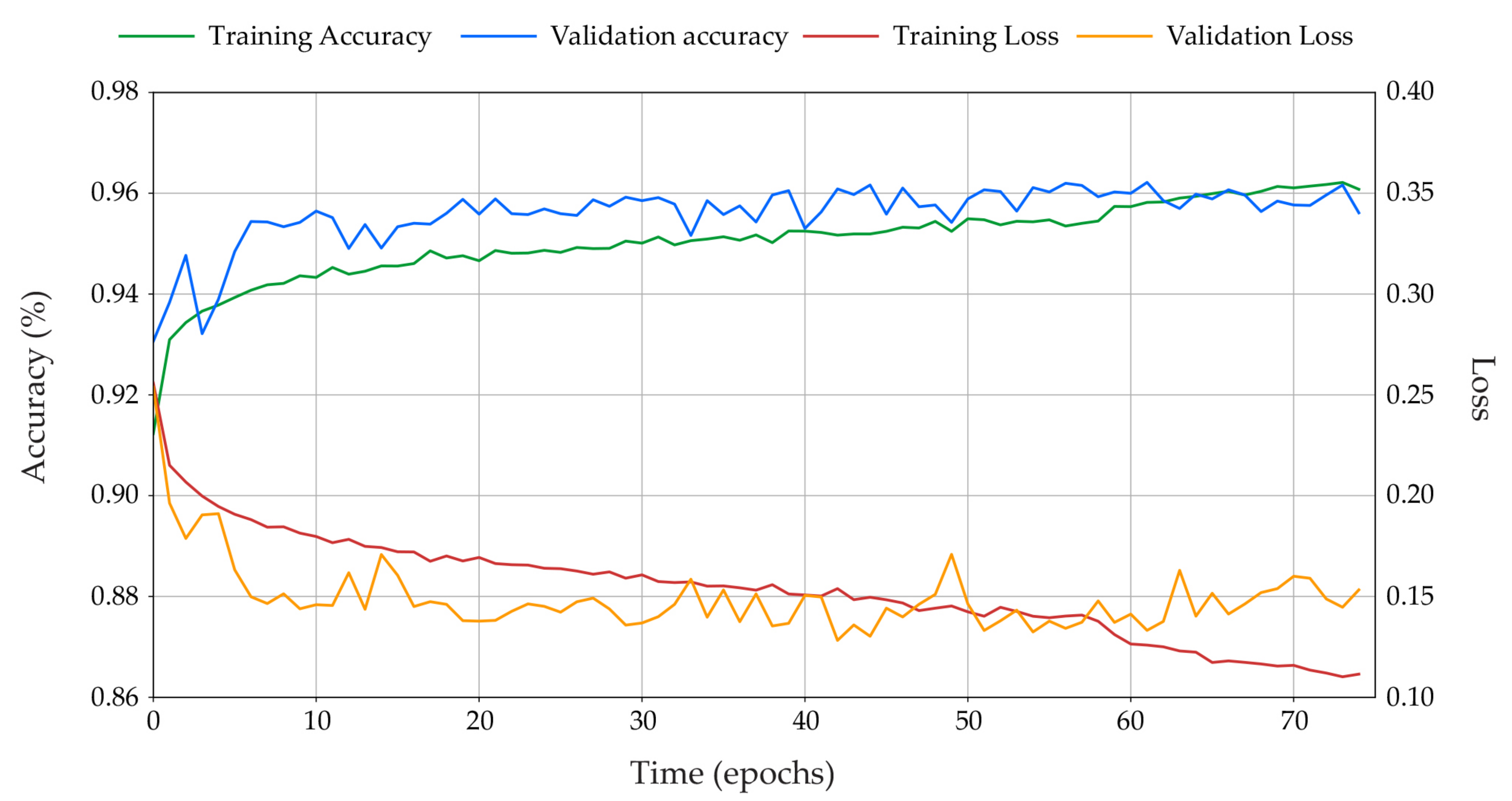
3.2. Deep Learning Training
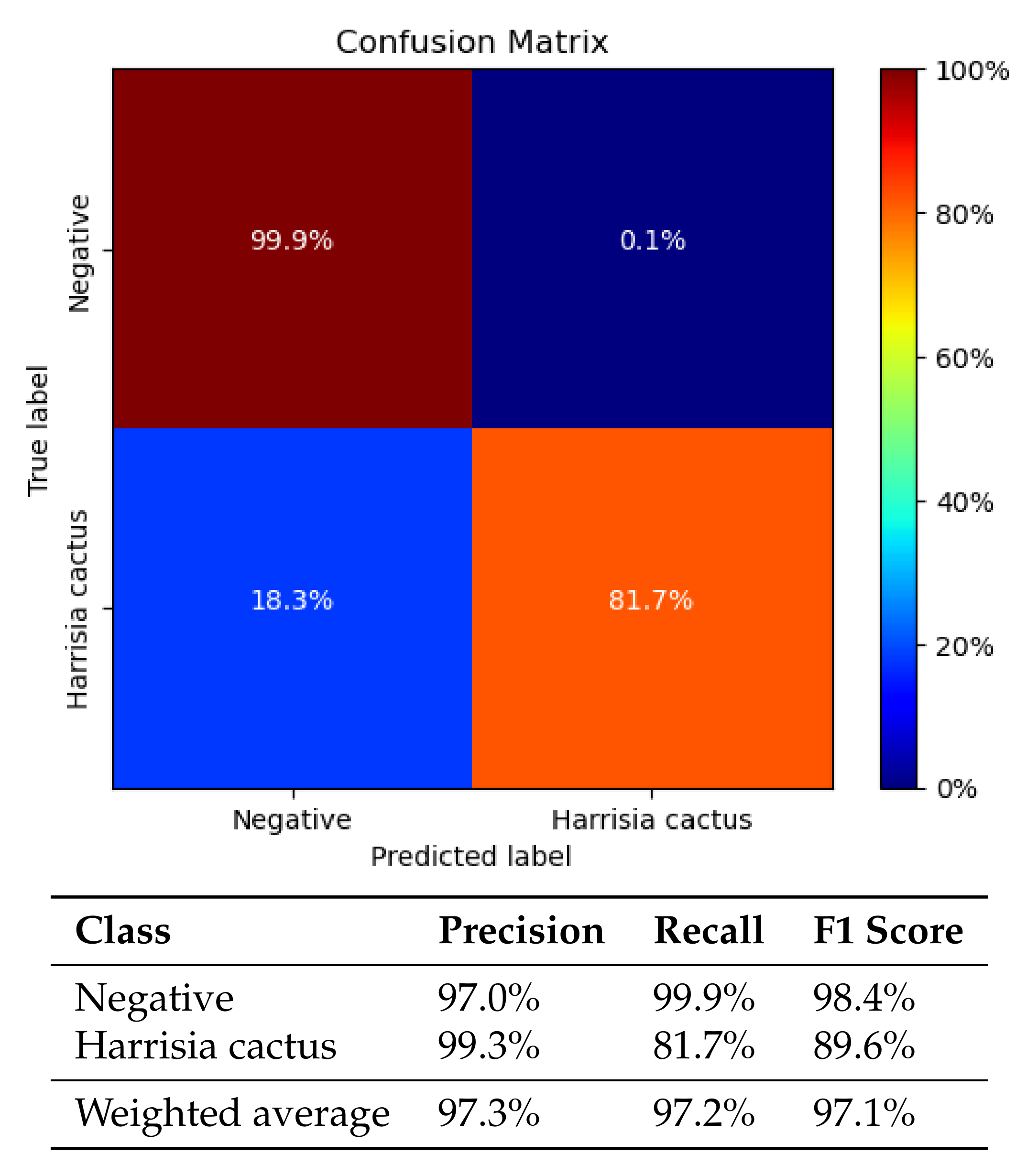
3.3. Classification Results
3.4. Spray Trial Results
3.4.1. Weed Knockdown
3.4.2. Herbicide Usage
3.4.3. Labour Requirement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFadyen, R.E. The cactus mealybug Hypogeococcus Festerianus [Hem.: Pseudococcidae] Agent Biol. Control Eriocereus Martinii[Cactaceae] Aust. Entomophaga 1979, 24, 281–287. [Google Scholar] [CrossRef]
- Houston, W.A.; Elder, R. Biocontrol of Harrisia cactus Harrisia Martinii Mealybug Hypogeococcus Festerianus (Hemiptera: Pseudococcidae) Salt-Influ. Habitats Aust. Austral Entomol. 2019, 58, 696–703. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Hoskins, A.J.; Haubrock, P.J.; Cuthbert, R.N.; Diagne, C.; Leroy, B.; Andrews, L.; Page, B.; Cassey, P.; Sheppard, A.W.; et al. Detailed assessment of the reported economic costs of invasive species in Australia. NeoBiota 2021, 67, 511–550. [Google Scholar] [CrossRef]
- Department of Agriculture and Fisheries. Harrisia Cactus Fact Sheet 2020. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0003/49179/harrisia-cactus.pdf (accessed on 30 August 2021).
- McFadyen, R.E. Harrisia (Eriocereus) martinii (Labour.) Britton—Harrisia cactus Acanthocereus tetragonus (L.) Hummelink—Sword Pear. In Biological Control of Weeds in Australia; CSIRO Publishing: Collingwood, VIC, Australia, 2012; pp. 274–281. [Google Scholar]
- McFadyen, R.E. The Ecology of Exotic Animals and Plants in Australia; John Wiley and Sons: Brisbane, QLD, Australia, 1986; Chapter Harrisia Cactus in Queensland; pp. 241–261. [Google Scholar]
- Duursma, D.E.; Gallagher, R.V.; Roger, E.; Hughes, L.; Downey, P.O.; Leishman, M.R. Next-Generation Invaders? Hotspots for Naturalised Sleeper Weeds in Australia under Future Climates. PLoS ONE 2013, 8, e84222. [Google Scholar] [CrossRef] [PubMed]
- Duursma, D.E.; Gallagher, R.V.; Roger, E.; Hughes, L.; Downey, P.O.; Leishman, M.R. Determining Current and Future Weed Threads in Australia. Available online: http://www.weedfutures.net/species.php?id=1094 (accessed on 28 August 2021).
- Atlas of Living Australia. Species Page and Occurence Records for Harrisia martinii. Available online: https://doi.org/10.26197/ala.6a4d4c11-a4bb-4b84-aa67-31168690b536 (accessed on 30 May 2021).
- McFadyen, R.E. The Harrisia Cactus Eradication Scheme: Policy Making by Technocrats. Master’s Thesis, University of Queensland, Brisbane, QLD, Australia, 1991. [Google Scholar]
- Townsend, S. Harrisia Cactus is Moving South, Reaches Narrabri; The Northern Daily Leader: Tamworth, NSW, Australia; Available online: https://www.northerndailyleader.com.au/story/6320031/watch-out-harrisia-cactus-is-on-the-move-south/?cs=159 (accessed on 28 August 2021).
- Croplands. WEEDit Optical Spot Spraying. Available online: https://croplands.com/au/products/weed-it-optical-spot-spraying/ (accessed on 28 August 2021).
- Trimble. WeedSeeker Spot Spray System. Available online: https://agriculture.trimble.com/product/weedseeker-spot-spray-system/ (accessed on 28 August 2021).
- Rural Directions Pty Ltd. Case Study: Optical Spot Spraying; Technical Report; Grains Research and Development Corporation (GRDC): Clare, SA, Australia, 2019. [Google Scholar]
- Hu, K.; Wang, Z.; Coleman, G.; Bender, A.; Yao, T.; Zeng, S.; Song, D.; Schumann, A.; Walsh, M. Deep Learning Techniques for In-Crop Weed Identification: A Review. arXiv 2021, arXiv:2103.14872. [Google Scholar]
- Olsen, A. Improving the Accuracy of Weed Species Detection for Robotic Weed Control in Complex Real-Time Environments. Ph.D. Thesis, James Cook University, Townsville, QLD, Australia, 2020. [Google Scholar]
- Calvert, B.; Olsen, A.; Philippa, B.; Azghadi, M.R. AutoWeed: Detecting Harrisia Cactus in the Goondiwindi Region For Selective Spot-Spraying. In Proceedings of the 1st Queensland Pest Animal and Weed Symposium; Sydes, T., Ed.; Weed Society of Queensland Pty. Ltd.: Toowoomba, QLD, Australia, 2019; p. 52. [Google Scholar]
- Olsen, A.; Konovalov, D.A.; Philippa, B.; Ridd, P.; Wood, J.C.; Johns, J.; Banks, W.; Girgenti, B.; Kenny, O.; Whinney, J.; et al. DeepWeeds: A Multiclass Weed Species Image Dataset for Deep Learning. Sci. Rep. 2019, 9, 2058. [Google Scholar] [CrossRef] [PubMed]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.S.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef] [Green Version]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 25, 1097–1105. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Lammie, C.; Olsen, A.; Carrick, T.; Rahimi Azghadi, M. Low-Power and High-Speed Deep FPGA Inference Engines for Weed Classification at the Edge. IEEE Access 2019, 7, 51171–51184. [Google Scholar] [CrossRef]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.C. MobileNetV2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar] [CrossRef] [Green Version]
- Yosinski, J.; Clune, J.; Bengio, Y.; Lipson, H. How transferable are features in deep neural networks? arXiv 2014, arXiv:1411.1792. [Google Scholar]
- North West Local Land Services. Harrisia Cactus Herbicide Trials Project from March 2015 to March 2017; Technical Report; North West Local Land Services: Boggabilla, NSW, Australia, 2017. [Google Scholar]
- Zhou, B.; Khosla, A.; Lapedriza, À.; Oliva, A.; Torralba, A. Learning Deep Features for Discriminative Localization. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]






| Product | Description | Volume (per 100 L Mixture) | Price (per 100 L Mixture) |
|---|---|---|---|
| Grazon Extra | Group I herbicide | 300 mL | $10.49 |
| Activator Surfactant | Wetting agent | 300 mL | $2.08 |
| Associate | Group B herbicide | 5 g | $0.40 |
| Red dye | Spray marker | 75 mL | - |
| Harrisia Cactus | Negatives | Total Images | |
|---|---|---|---|
| Training | 6226 | 40,296 | 46,522 |
| Validation | 1556 | 10,075 | 11,631 |
| Total | 7782 | 50,371 | 58,153 |
| Plot ID | Weed Density | Control Method | Time Taken (mins) | Chemical Used (L) | Estimated Weed Count | Estimated Weeds Killed (%) | Estimated Weeds Missed (%) |
|---|---|---|---|---|---|---|---|
| A | Low | High volume spot spray | 14 | 35 | <100 | 96 | 4 |
| B | Medium | High volume spot spray | 15 | 65 | >200 | 96 | 2 |
| C | High | High volume spot spray | 16 | 125 | >400 | 97 | 0.75 |
| D | Low | Hand spray | 25 | 75 | <100 | 100 | 0 |
| E | Low | Hand spray | 25 | 65 | >100 | 97 | 3 |
| F | High | Hand spray | 48 | 148 | >400 | 98 | 1.25 |
| G | Low | Low volume spot spray | 11 | 40 | >100 | 93 | 3 |
| H | Medium | Low volume spot spray | 17 | 65 | >200 | 96 | 2.5 |
| I | High | Low volume spot spray | 13 | 75 | >300 | 96 | 0 |
| Control Method | Average Weed Knockdown (%) |
|---|---|
| High volume spot spray | |
| Hand spray | |
| Low volume spot spray |
| Control Method | Average Missed (%) | Spraying Accuracy (%) |
|---|---|---|
| High volume spot spray | ||
| Hand spray | ||
| Low volume spot spray |
| Control Method | Weed Density | Herbicide Used (L) | Normalised Herbicide |
|---|---|---|---|
| High volume spot spray | Low | 35 | 0.46 |
| Medium | 65 | 1 | |
| High | 125 | 0.86 | |
| Hand spray | Low | 75 | 1 |
| Low | 65 | 1 | |
| High | 145 | 1 | |
| Low volume spot spray | Low | 40 | 0.53 |
| Medium | 65 | 1 | |
| High | 75 | 0.52 |
| Control Method | Usage (L/ha) | Cost ($/ha) |
|---|---|---|
| Boom spray high volume | ||
| Hand spray | ||
| Boom spray low volume |
| Control Method | Weed Density | Time Taken (mins) | Normalised Time |
|---|---|---|---|
| High volume spot spray | Low | 14 | 0.56 |
| Medium | 15 | 0.6 | |
| High | 16 | 0.33 | |
| Hand spray | Low | 25 | 1 |
| Low | 25 | 1 | |
| High | 48 | 1 | |
| Low volume spot spray | Low | 11 | 0.44 |
| Medium | 17 | 0.68 | |
| High | 13 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvert, B.; Olsen, A.; Whinney, J.; Rahimi Azghadi, M. Robotic Spot Spraying of Harrisia Cactus (Harrisia martinii) in Grazing Pastures of the Australian Rangelands. Plants 2021, 10, 2054. https://doi.org/10.3390/plants10102054
Calvert B, Olsen A, Whinney J, Rahimi Azghadi M. Robotic Spot Spraying of Harrisia Cactus (Harrisia martinii) in Grazing Pastures of the Australian Rangelands. Plants. 2021; 10(10):2054. https://doi.org/10.3390/plants10102054
Chicago/Turabian StyleCalvert, Brendan, Alex Olsen, James Whinney, and Mostafa Rahimi Azghadi. 2021. "Robotic Spot Spraying of Harrisia Cactus (Harrisia martinii) in Grazing Pastures of the Australian Rangelands" Plants 10, no. 10: 2054. https://doi.org/10.3390/plants10102054






