Evaluation of the Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantations in ALS Patients by Investigating Patients’ Specific Immunological and Biochemical Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Immunological Factor Estimation
2.3. Biochemical Evaluation
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characterization and Safety Evaluation
3.2. Immunological Assessment
3.2.1. Tumor Necrosis Factor-Alpha (TNF-α) Levels
3.2.2. Neurofilament Light Chain (NFL) Levels
3.2.3. Glial-Cell-Derived Neurotrophic Factor (GDNF) Levels
3.3. Biochemical Evaluation
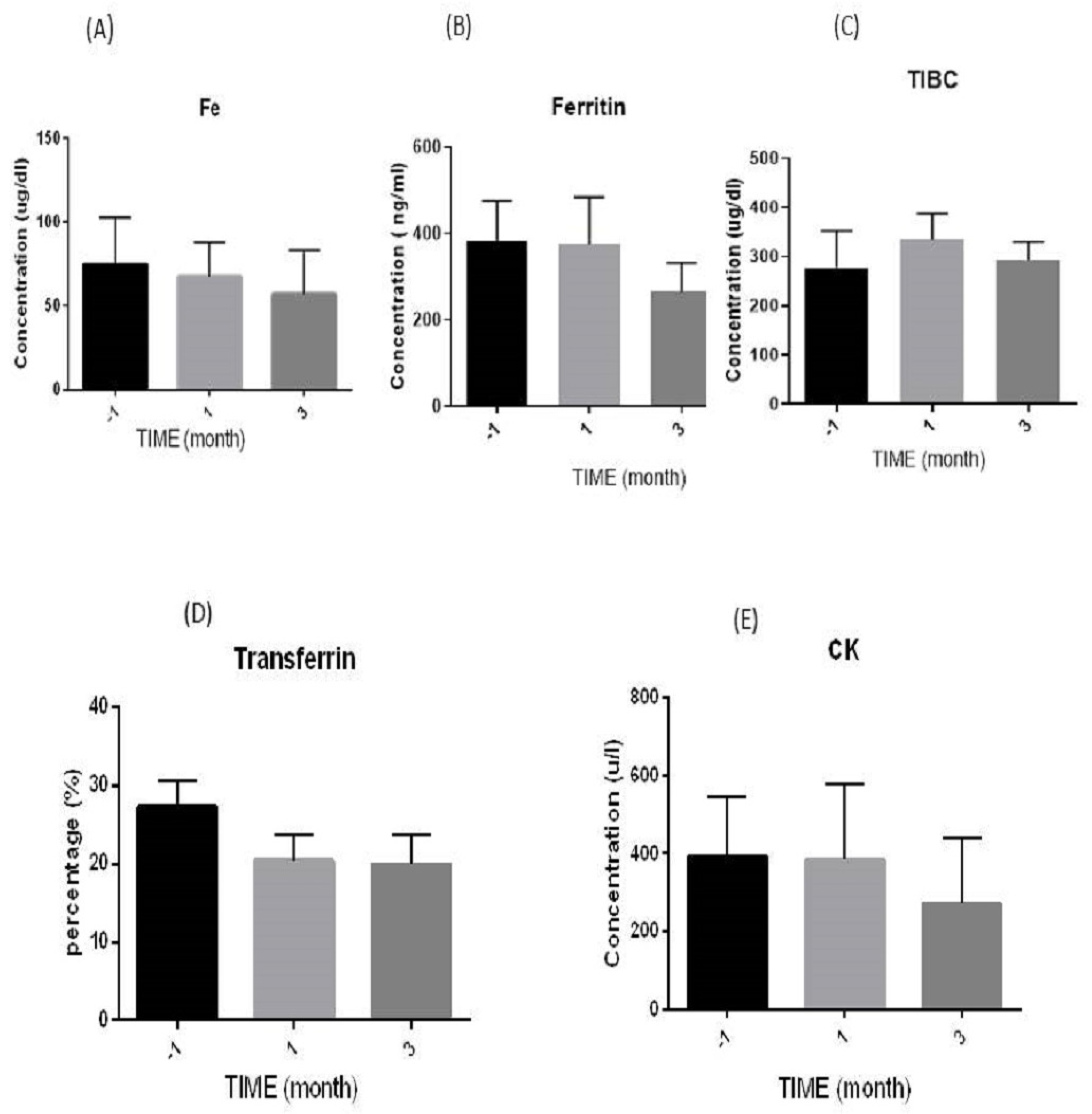
3.3.1. Iron (Fe) Levels
3.3.2. Ferritin Levels
3.3.3. Total Iron Binding Capacity (TIBC) Levels
3.3.4. Transferrin Levels
3.3.5. Creatine Kinase (CK) Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- ALS Managed Care ConsiderationsSantaniello, Briana. Am. J. Manag. Care 2018, 24, S336–S341.
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.-X. Mutations in Cu/Zn Superoxide Dismutase Gene Are Associated with Familial Amyotrophic Lateral Sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski Jr, T.J.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T. Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E. TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic Lateral Sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Benatar, M.; Boylan, K.; Jeromin, A.; Rutkove, S.B.; Berry, J.; Atassi, N.; Bruijn, L. ALS Biomarkers for Therapy Development: State of the Field and Future Directions. Muscle Nerve 2016, 53, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Vescovi, A.; Cantello, R.; Gelati, M.; Vercelli, A. Stem Cells Therapy for ALS. Expert Opin. Biol. Ther. 2016, 16, 187–199. [Google Scholar] [CrossRef]
- Faravelli, I.; Riboldi, G.; Nizzardo, M.; Simone, C.; Zanetta, C.; Bresolin, N.; Comi, G.P.; Corti, S. Stem Cell Transplantation for Amyotrophic Lateral Sclerosis: Therapeutic Potential and Perspectives on Clinical Translation. Cell. Mol. Life Sci. 2014, 71, 3257–3268. [Google Scholar] [CrossRef]
- Lo Coco, D.; Marchese, S.; La Bella, V.; Piccoli, T.; Lo Coco, A. The Amyotrophic Lateral Sclerosis Functional Rating Scale Predicts Survival Time in Amyotrophic Lateral Sclerosis Patients on Invasive Mechanical Ventilation. Chest 2007, 132, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Czaplinski, A.; Yen, A.A.; Appel, S.H. Forced Vital Capacity (FVC) as an Indicator of Survival and Disease Progression in an ALS Clinic Population. J. Neurol. Neurosurg. Psychiatry 2006, 77, 390–392. [Google Scholar] [CrossRef]
- Tavakol-Afshari, J.; Boroumand, A.R.; Farkhad, N.K.; Adhami Moghadam, A.; Sahab-Negah, S.; Gorji, A. Safety and Efficacy of Bone Marrow Derived-Mesenchymal Stem Cells Transplantation in Patients with Amyotrophic Lateral Sclerosis. Regen. Ther. 2021, 18, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Longone, P.; Spalloni, A.; Pieroni, L.; Urbani, A. Crosstalk Between Oxidative Stress and Mitochondrial Damage: Focus on Amyotrophic Lateral Sclerosis. In Mitochondria in Health and in Sickness; Urbani, A., Babu, M., Eds.; Springer: Singapore, 2019; pp. 71–82. ISBN 978-981-13-8367-0. [Google Scholar] [CrossRef]
- Valko, K.; Ciesla, L. Amyotrophic Lateral Sclerosis. In Progress in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 58, pp. 63–117. ISBN 0079-6468. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Bowers, W.J. Tumor Necrosis Factor-Alpha and the Roles It Plays in Homeostatic and Degenerative Processes within the Central Nervous System. J. Neuroimmune Pharmacol. 2012, 7, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhang, J.; Wang, D.; Zhao, F.; Cao, Z.; Aschner, M.; Luo, W. The Role of Autophagy in Modulation of Neuroinflammation in Microglia. Neuroscience 2016, 319, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Olmos, G.; Lladó, J. Tumor Necrosis Factor Alpha: A Link between Neuroinflammation and Excitotoxicity. Mediators Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Sainio, M.T.; Rasila, T.; Molchanova, S.M.; Järvilehto, J.; Torregrosa-Muñumer, R.; Harjuhaahto, S.; Pennonen, J.; Huber, N.; Herukka, S.-K.; Haapasalo, A. Neurofilament Light Regulates Axon Caliber, Synaptic Activity, and Organelle Trafficking in Cultured Human Motor Neurons. Front. Cell Dev. Biol. 2022, 9, 820105. [Google Scholar] [CrossRef]
- Mariotto, S.; Farinazzo, A.; Magliozzi, R.; Alberti, D.; Monaco, S.; Ferrari, S. Serum and Cerebrospinal Neurofilament Light Chain Levels in Patients with Acquired Peripheral Neuropathies. J. Peripher. Nerv. Syst. 2018, 23, 174–177. [Google Scholar] [CrossRef]
- Barua, S.; Pathak, Y. V Unilateral Ex Vivo Gene Therapy by GDNF in Neurodegenerative Diseases. In Gene Delivery Systems; CRC Press: Boca Raton, FL, USA, 2022; pp. 155–161. ISBN 1003186068. [Google Scholar]
- Hellmich, H.L.; Kos, L.; Cho, E.S.; Mahon, K.A.; Zimmer, A. Embryonic Expression of Glial Cell-Line Derived Neurotrophic Factor (GDNF) Suggests Multiple Developmental Roles in Neural Differentiation and Epithelial-Mesenchymal Interactions. Mech. Dev. 1996, 54, 95–105. [Google Scholar] [CrossRef]
- Zhao, Y.; Haney, M.J.; Jin, Y.S.; Uvarov, O.; Vinod, N.; Lee, Y.Z.; Langworthy, B.; Fine, J.P.; Rodriguez, M.; El-Hage, N. GDNF-Expressing Macrophages Restore Motor Functions at a Severe Late-Stage, and Produce Long-Term Neuroprotective Effects at an Early-Stage of Parkinson’s Disease in Transgenic Parkin Q311X (A) Mice. J. Control. Release 2019, 315, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kokić, A.N.; Stević, Z.; Stojanović, S.; Blagojević, D.P.; Jones, D.R.; Pavlović, S.; Niketić, V.; Apostolski, S.; Spasić, M.B. Biotransformation of Nitric Oxide in the Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Redox Rep. 2005, 10, 265–270. [Google Scholar] [CrossRef]
- Halon, M.; Kaczor, J.J.; Ziolkowski, W.; Flis, D.J.; Borkowska, A.; Popowska, U.; Nyka, W.; Wozniak, M.; Antosiewicz, J. Changes in Skeletal Muscle Iron Metabolism Outpace Amyotrophic Lateral Sclerosis Onset in Transgenic Rats Bearing the G93A HmSOD1 Gene Mutation. Free Radic. Res. 2014, 48, 1363–1370. [Google Scholar] [CrossRef]
- Crichton, R.R.; Dexter, D.T.; Ward, R.J. Brain Iron Metabolism and Its Perturbation in Neurological Diseases. In Metal Ions in Neurological Systems; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–15. [Google Scholar] [CrossRef]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox Metals Homeostasis in Multiple Sclerosis and Amyotrophic Lateral Sclerosis: A Review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, G.; Badrick, T. Is Total Iron Binding Capacity (TIBC) Calculation Correct? Pathology 2019, 51, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Nigel Leigh, P. Amyotrophic Lateral Sclerosis. Orphanet J. Rare Dis. 2009, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Approach to Asymptomatic Creatine Kinase Elevation. Cleve. Clin. J. Med. 2016, 83, 37. [Google Scholar] [CrossRef]
- Amrit, A.N.; Anderson, M.S. Serum Creatine Phosphokinase in Amyotrophic Lateral Sclerosis: Correlation with Sex, Duration, and Skeletal Muscle Biopsy. Neurology 1974, 24, 834. [Google Scholar] [CrossRef]
- Chen, X.-P.; Wei, Q.-Q.; Ou, R.-W.; Hou, Y.-B.; Zhang, L.-Y.; Yuan, X.-Q.; Yao, Y.-Q.; Jia, D.-S.; Zhang, Q.; Li, W.-X. Creatine Kinase in the Diagnosis and Prognostic Prediction of Amyotrophic Lateral Sclerosis: A Retrospective Case-Control Study. Neural Regen. Res. 2021, 16, 591. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial Revisited: Revised Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.; Mukkamalla, S.K.R. Iron Binding Capacity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Koerper, M.A.; Dallman, P.R. Serum Iron Concentration and Transferrin Saturation in the Diagnosis of Iron Deficiency in Children: Normal Developmental Changes. J. Pediatr. 1977, 91, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Colpo, G.D.; Ascoli, B.M.; Wollenhaupt-Aguiar, B.; Pfaffenseller, B.; Silva, E.G.; Cirne-Lima, E.O.; Quevedo, J.; Kapczinski, F.; Rosa, A.R. Mesenchymal Stem Cells for the Treatment of Neurodegenerative and Psychiatric Disorders. Anais Da Academia Brasileira de Ciências 2015, 87, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.A.; Pereira-Rufino, L.D.S.; Vissoto, T.C.S.; Kerkis, I.; Neves, A.D.C.; da Silva, M.C.P. The Immunomodulatory Potential Role of Mesenchymal Stem Cells in Diseases of the Central Nervous System. Neurodegener. Dis. 2022, 22, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Guo, H.; Li, H.; Zhai, Y.; Gong, Z.; Wu, J.; Liu, J.; Dong, Y.; Hou, S.; Liu, J. RVG-Modified Exosomes Derived from Mesenchymal Stem Cells Rescue Memory Deficits by Regulating Inflammatory Responses in a Mouse Model of Alzheimer’s Disease. Immun. Ageing 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.C.F.; Repic, M.; Knoblich, J.A. Proliferation Control in Neural Stem and Progenitor Cells. Nat. Rev. Neurosci. 2015, 16, 647–659. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.; Wang, Y.; Gu, G.; Zhang, W.; Xia, R. Neural Stem Cell Transplantation Decreases Neuroinflammation in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurochem. 2016, 136, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Fratta, P.; Sharma, N.; Sidle, K.; Howard, R. Systemic Inflammatory Response and Neuromuscular Involvement in Amyotrophic Lateral Sclerosis. Neurol. Neuroinflamm. 2016, 3, e244. [Google Scholar] [CrossRef] [PubMed]
- Cudkowicz, M.E.; Lindborg, S.R.; Goyal, N.A.; Miller, R.G.; Burford, M.J.; Berry, J.D.; Nicholson, K.A.; Mozaffar, T.; Katz, J.S.; Jenkins, L.J. A Randomized Placebo-controlled Phase 3 Study of Mesenchymal Stem Cells Induced to Secrete High Levels of Neurotrophic Factors in Amyotrophic Lateral Sclerosis. Muscle Nerve 2022, 65, 291–302. [Google Scholar] [CrossRef]
- Berry, J.D.; Cudkowicz, M.E.; Windebank, A.J.; Staff, N.P.; Owegi, M.; Nicholson, K.; McKenna-Yasek, D.; Levy, Y.S.; Abramov, N.; Kaspi, H. NurOwn, Phase 2, Randomized, Clinical Trial in Patients with ALS: Safety, Clinical, and Biomarker Results. Neurology 2019, 93, e2294–e2305. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Dong, S.; Yang, W.; Qian, T.; Liu, X.; Cheng, Q.; Wang, J.; Chen, X. Role of Blood Neurofilaments in the Prognosis of Amyotrophic Lateral Sclerosis: A Meta-Analysis. Front. Neurol. 2021, 12, 712245. [Google Scholar] [CrossRef] [PubMed]
- Feneberg, E.; Oeckl, P.; Steinacker, P.; Verde, F.; Barro, C.; Van Damme, P.; Gray, E.; Grosskreutz, J.; Jardel, C.; Kuhle, J. Multicenter Evaluation of Neurofilaments in Early Symptom Onset Amyotrophic Lateral Sclerosis. Neurology 2018, 90, e22–e30. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, X.; Li, S.; Yang, F.; Wang, H.; Cui, F.; Huang, X. CSF Neurofilament Light Chain Elevation Predicts ALS Severity and Progression. Front. Neurol. 2020, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Lublin, F.D.; Lock, C.; Pelletier, D.; Chitnis, T.; Mehra, M.; Gothelf, Y.; Aricha, R.; Lindborg, S.; Lebovits, C. Evaluation of Neurotrophic Factor Secreting Mesenchymal Stem Cells in Progressive Multiple Sclerosis. Mult. Scler. J. 2023, 29, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N. Beneficial Effects of Autologous Mesenchymal Stem Cell Transplantation in Active Progressive Multiple Sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, C.; Bonilla, S.; Tabares, L.; Martínez, S. Neuroprotective Effect of Adult Hematopoietic Stem Cells in a Mouse Model of Motoneuron Degeneration. Neurobiol. Dis. 2007, 26, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Garbuzova-Davis, S.; Borlongan, C.V. Patching Up the Permeability: The Role of Stem Cells in Lessening Neurovascular Damage in Amyotrophic Lateral Sclerosis. Stem Cells Transl. Med. 2022, 11, 1196–1209. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Shang, H. Aberrations of Biochemical Indicators in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Transl. Neurodegener. 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.M.; Simmons, Z.; Beard, J.L.; Stephens, H.E.; Connor, J.R. Plasma Biomarkers Associated with ALS and Their Relationship to Iron Homeostasis. Muscle Nerve 2010, 42, 95–103. [Google Scholar] [CrossRef]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A. Patterns of Levels of Biological Metals in CSF Differ among Neurodegenerative Diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef]
- Moghadam, M.S.; Azimian, H.; Afshari, J.T.; Toossi, M.T.B.; Farkhad, N.K.; Aghaee-Bakhtiari, S.H. Chromosomal Instability in Various Generations of Human Mesenchymal Stem Cells Following the Therapeutic Radiation Doses. Stem Cells Int. 2023, 2023, 9991656. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Chen, X.; Li, S.; Shang, H. Abnormal Serum Iron-Status Indicator Changes in Amyotrophic Lateral Sclerosis (ALS) Patients: A Meta-Analysis. Front. Neurol. 2020, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Moreau, C.; Kyheng, M.; Garçon, G.; Rolland, A.S.; Blasco, H.; Gelé, P.; Timothée Lenglet, T.; Veyrat-Durebex, C.; Corcia, P. A Ferroptosis–Based Panel of Prognostic Biomarkers for Amyotrophic Lateral Sclerosis. Sci. Rep. 2019, 9, 2918. [Google Scholar] [CrossRef] [PubMed]
- Goodall, E.F.; Haque, M.S.; Morrison, K.E. Increased Serum Ferritin Levels in Amyotrophic Lateral Sclerosis (ALS) Patients. J. Neurol. 2008, 255, 1652–1656. [Google Scholar] [CrossRef]
- Monov, D.; Molodozhnikova, N. Biochemical Parameters as a Tool to Assess the Nutritional Status of Patients with Amyotrophic Lateral Sclerosis. Front. Neurol. 2024, 14, 1258224. [Google Scholar] [CrossRef]
- Hertel, N.; Kuzma-Kozakiewicz, M.; Gromicho, M.; Grosskreutz, J.; de Carvalho, M.; Uysal, H.; Dengler, R.; Petri, S.; Körner, S. Analysis of Routine Blood Parameters in Patients with Amyotrophic Lateral Sclerosis and Evaluation of a Possible Correlation with Disease Progression—A Multicenter Study. Front. Neurol. 2022, 13, 940375. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhazaali-Ali, Z.; Sahab-Negah, S.; Boroumand, A.R.; Farkhad, N.K.; Khodadoust, M.A.; Tavakol-Afshari, J. Evaluation of the Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantations in ALS Patients by Investigating Patients’ Specific Immunological and Biochemical Biomarkers. Diseases 2024, 12, 99. https://doi.org/10.3390/diseases12050099
Alkhazaali-Ali Z, Sahab-Negah S, Boroumand AR, Farkhad NK, Khodadoust MA, Tavakol-Afshari J. Evaluation of the Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantations in ALS Patients by Investigating Patients’ Specific Immunological and Biochemical Biomarkers. Diseases. 2024; 12(5):99. https://doi.org/10.3390/diseases12050099
Chicago/Turabian StyleAlkhazaali-Ali, Zahraa, Sajad Sahab-Negah, Amir Reza Boroumand, Najmeh Kaffash Farkhad, Mohammad Ali Khodadoust, and Jalil Tavakol-Afshari. 2024. "Evaluation of the Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantations in ALS Patients by Investigating Patients’ Specific Immunological and Biochemical Biomarkers" Diseases 12, no. 5: 99. https://doi.org/10.3390/diseases12050099





